Process for preparing nuclear-grade hafnium oxide and zirconium oxide through solvent extractive separation of zirconium and hafnium
A technology of hafnium oxide and zirconium oxide, which is applied in the chemical field, can solve problems such as complex processes, and achieve the effects of simplifying process flow, improving extraction efficiency, and reducing work intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
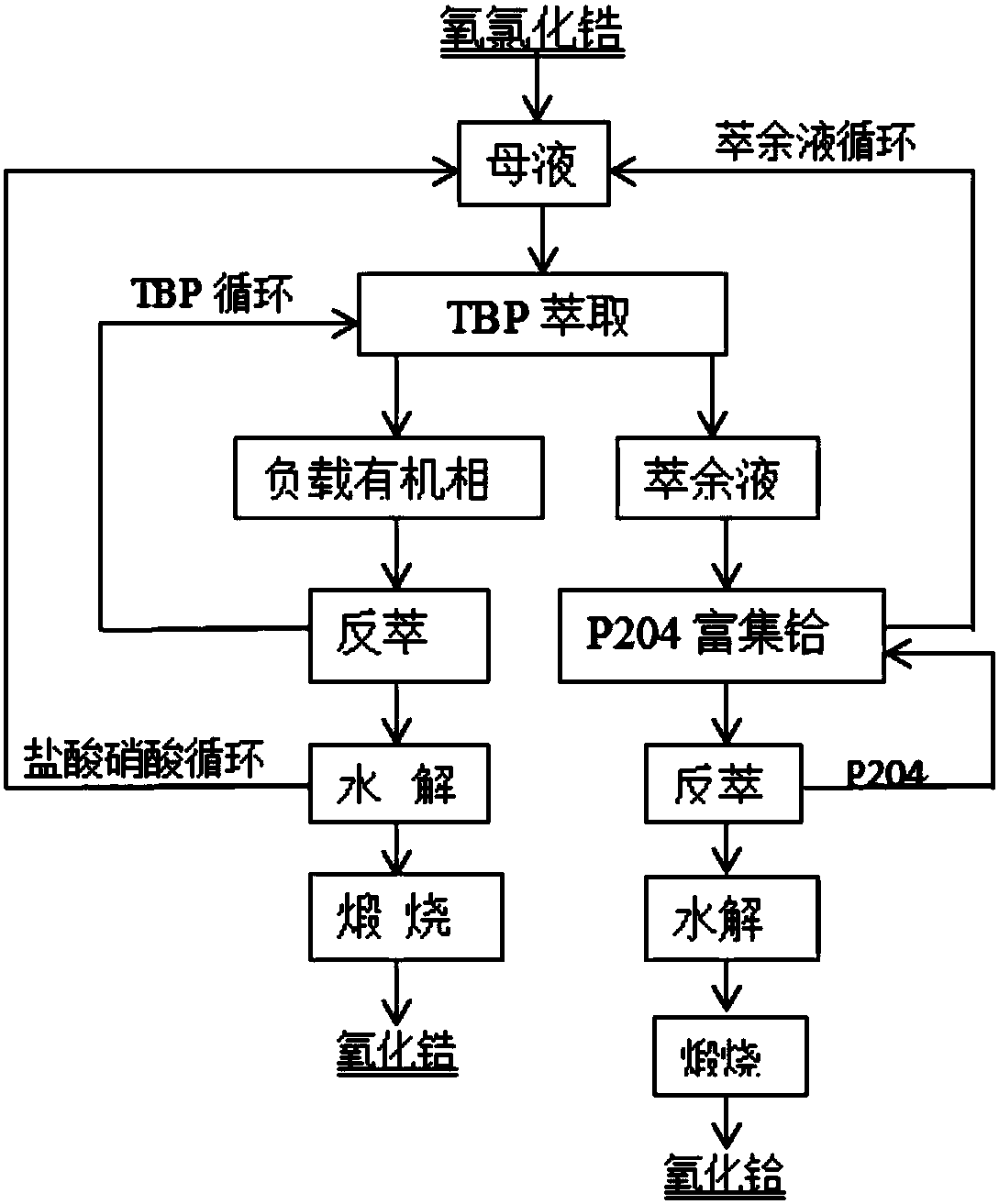
[0042] (1) Prepare 3molL -1 Acidification of nitric acid solution: Take 5L of concentrated nitric acid solution and add it to 20L of deionized water, stir and mix evenly. Prepare an organic phase containing 40% TBP and 10% ionic liquid by volume: take 10L TBP, 2.5L ionic liquid and 12.5L kerosene and mix them evenly. Prepare 1molL -1 Feed liquid: Take 6.45Kg of zirconium oxychloride, add deionized water to dissolve, add hydrochloric acid and nitric acid, make the solution acidity 4molL -1 , and finally set the volume to 20L. Preparation of 10% P204 organic phase: take 0.8L P204 and 7.2L kerosene and mix evenly. Preparation of 1.6L stripping solution: Dissolve 320g of ammonium bicarbonate in deionized water and dilute to 1.6L.
[0043] (2) First, the organic phase and the acidified nitric acid solution are compared in a ratio of 1:1, and the countercurrent acidification is carried out in a centrifugal extractor, and the organic phase is pretreated in three stages.
[0044]...
Embodiment 2
[0050] A method similar to that of Example 1 was used to prepare acid solutions, feed solutions and organic phases with different concentrations. The concentration of acidified nitric acid is 4molL -1 , in the organic phase, the volume fraction of TBP is 60%, the volume fraction of ionic liquid is 0%, and the acidity of feed liquid is 5molL -1 , the concentration of zirconium and hafnium is 1.5molL -1 . The specific operation method is similar to that of Example 1, the only difference is that the acidified organic phase is 2:1 compared with the feed liquid, and the number of extraction stages is 15 to obtain nuclear-grade zirconium dioxide and hafnium oxide, and the recovery rate of zirconium is greater than 90% %.
Embodiment 3
[0052] A method similar to that of Example 1 was used to prepare acid solutions, feed solutions and organic phases with different concentrations. The concentration of acidified nitric acid is 3molL -1 , in the organic phase, the volume fraction of TBP is 50%, the volume fraction of ionic liquid is 5%, and the acidity of feed liquid is 4molL -1 , the concentration of zirconium and hafnium is 0.5molL -1 . The specific operation method is similar to that of Example 1, the only difference is that the acidified organic phase is 1:1 compared with the feed liquid, and the number of extraction stages is 6 to obtain nuclear-grade zirconium dioxide and hafnium oxide, and the recovery rate of zirconium is greater than 90% %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com