Method for synthesizing high-selectivity oxaziridine through chemical enzyme process
An oxaziridine and high-selectivity technology is applied in the field of chemical-enzymatic synthesis of high-selectivity oxaziridine, and can solve the problems of harmful environment and cumbersome production process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Experimental instruments and reagents
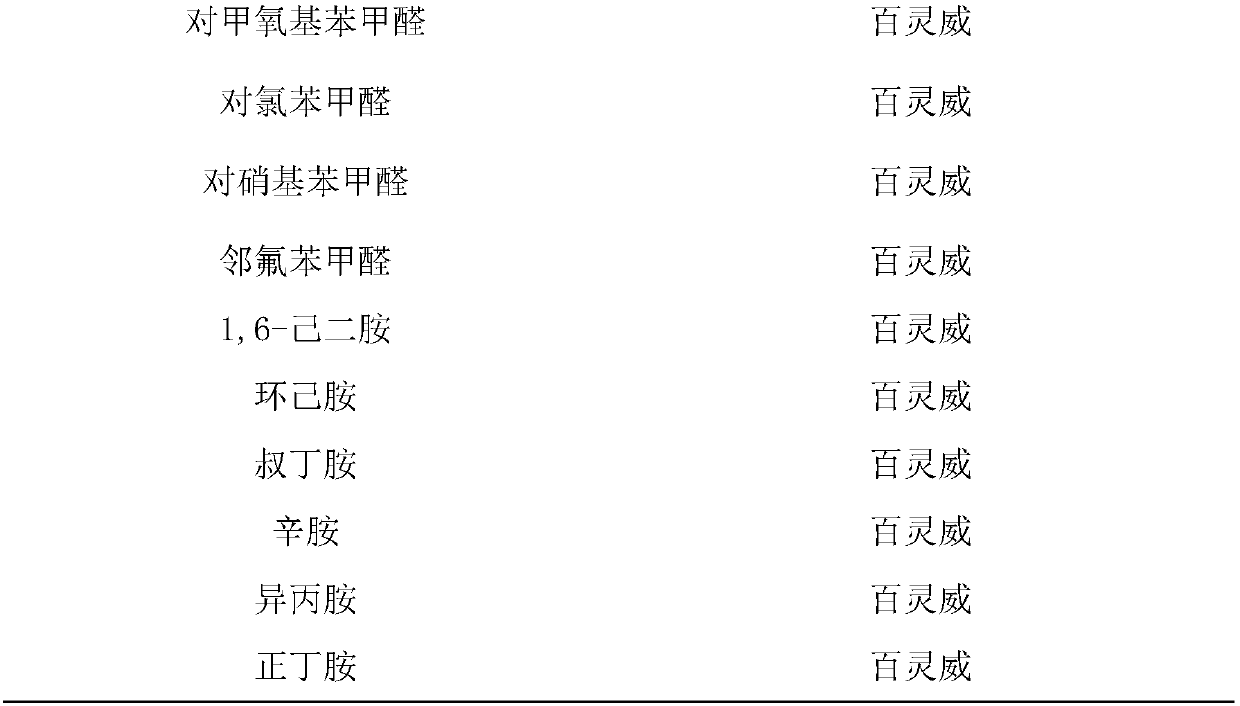
[0024] The experimental raw materials and instruments used in the embodiments of the present invention are shown in Table 1.
[0025] Table 1 Experimental Instruments and Reagents
[0026]
[0027]
[0028] a : Candida Antarctica lipase B (CALB, 10000U / mL) b : Novozyme 435 (the commercial immobilized CALB, 15000U / g)
[0029] 2. Determination of the best reaction conditions
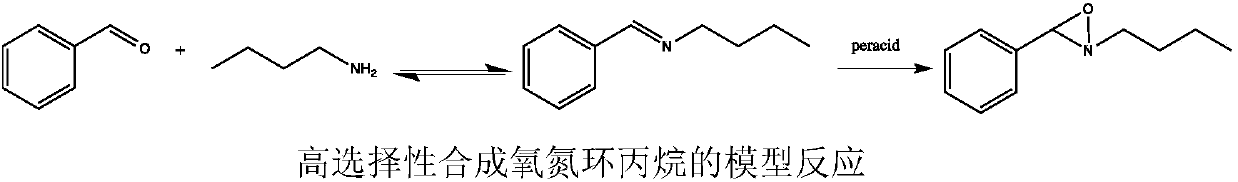
[0030] The oxidation reaction between benzaldehyde and n-butylamine was chosen as a model, and benzaldehyde, n-butylamine, lipase, oxidant, peroxyacid precursor and solvent were mixed in a round bottom flask, and the reaction was stirred at room temperature. Detect the reaction process with TLC, after the reaction finishes, the reaction mixture is extracted twice with ethyl acetate, and the organic layer is washed with anhydrous Na 2 SO 4 Dry and concentrate. Then it was further purified by silica gel column chromatography (ethyl acetate / hexane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com