Method for functionalization modification of benzyl hydrogen of benzyl-containing compound with C-C double bonds or carbonyl groups
A benzyl hydrogen functional, carbon-carbon double bond technology, applied in organic chemistry and other directions, can solve the problems of large environmental impact and high cost, and achieve the effects of short process, low cost and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
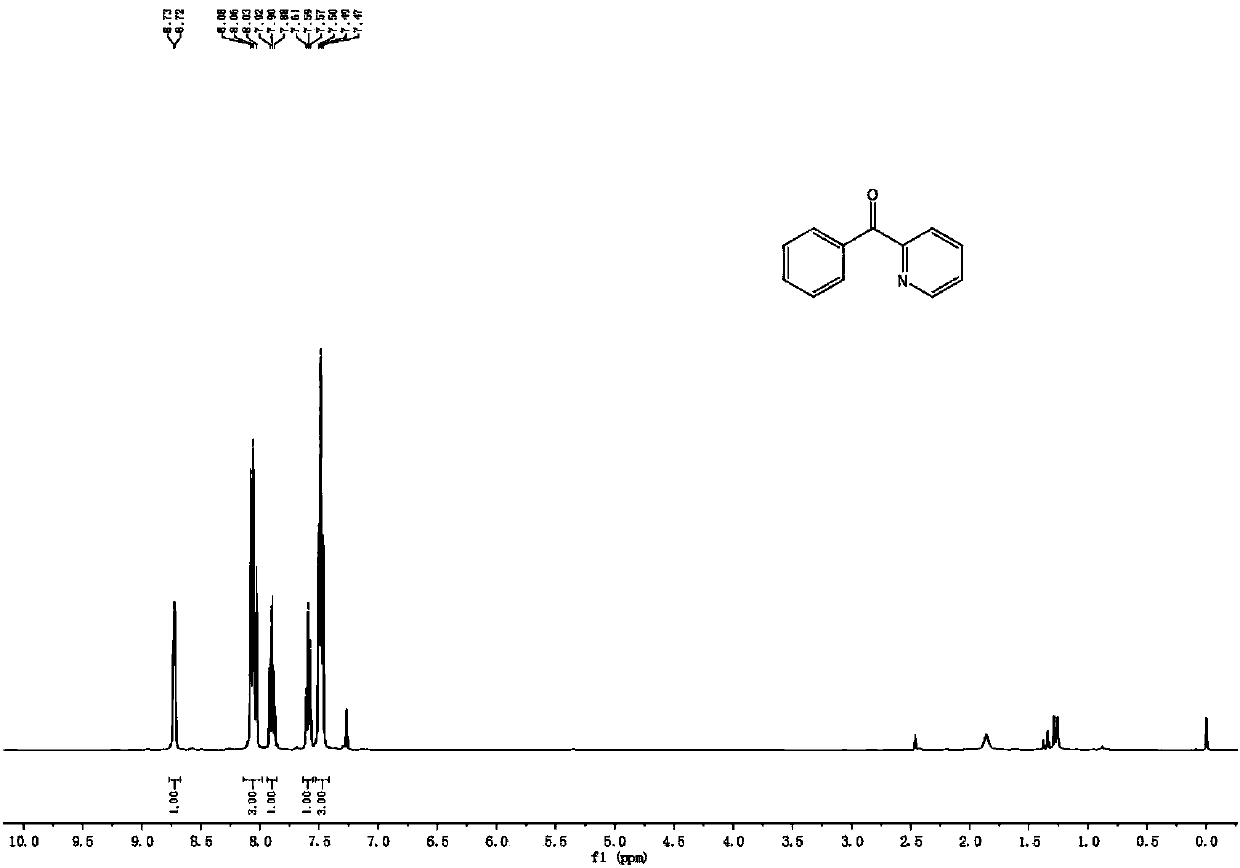
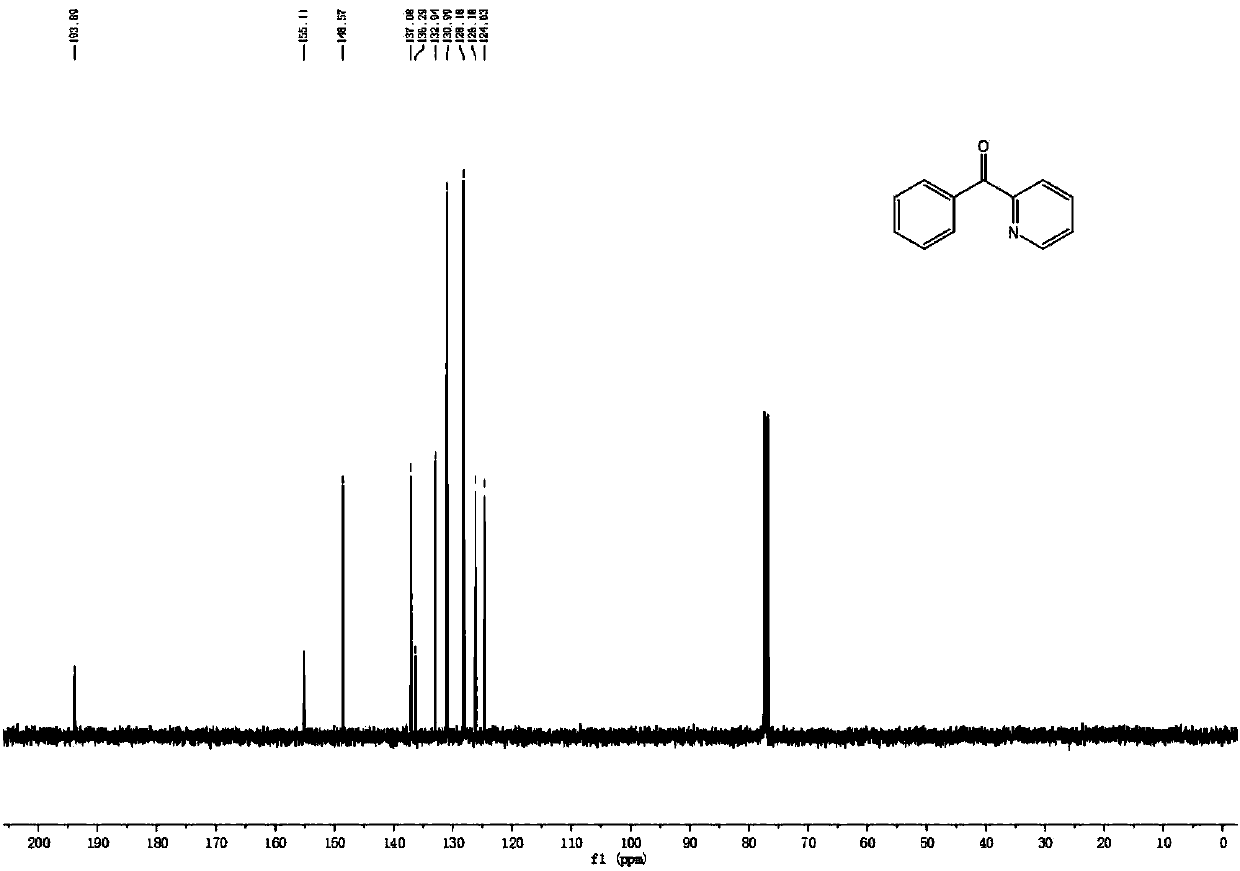
[0038] 2-benzylpyridine (0.5mmol), sodium methoxide (1mmol), K 2 S 2 o 8 (1mmol) and dimethyl sulfoxide (2mL) were added to the reactor, in the air environment, at a temperature of 120 ℃ for 12h, after the reaction was over, the excess dimethyl sulfoxide was recovered by distillation, and the mixture was separated by chromatographic column , to obtain 2-pyridyl phenyl ketone; yield 95%.
[0039] 1 H NMR (400MHz, CDCl 3 )δ8.72(d, J=4.6Hz, 1H), 8.14-7.98(m, 3H), 7.90(t, J=7.7Hz, 1H), 7.59(t, J=7.3Hz, 1H), 7.49( t, J=7.4Hz, 3H).
[0040] 13 C NMR (101MHz, CDCl 3 )δ193.9, 155.1, 148.5, 137.0, 136.3, 132.9, 130.9, 128.1, 126.1, 124.6.
Embodiment 2
[0042] 2-benzylpyridine (0.5mmol), sodium methoxide (1mmol), K 2 S 2 o 8 (1mmol) and dimethyl sulfoxide (2mL) were added to the reactor, in the air environment, at a temperature of 110 ° C for 16h, after the reaction, the excess dimethyl sulfoxide was recovered by distillation, and the mixture was separated by chromatography , to obtain 2-pyridyl phenyl ketone; yield 91%.
Embodiment 3
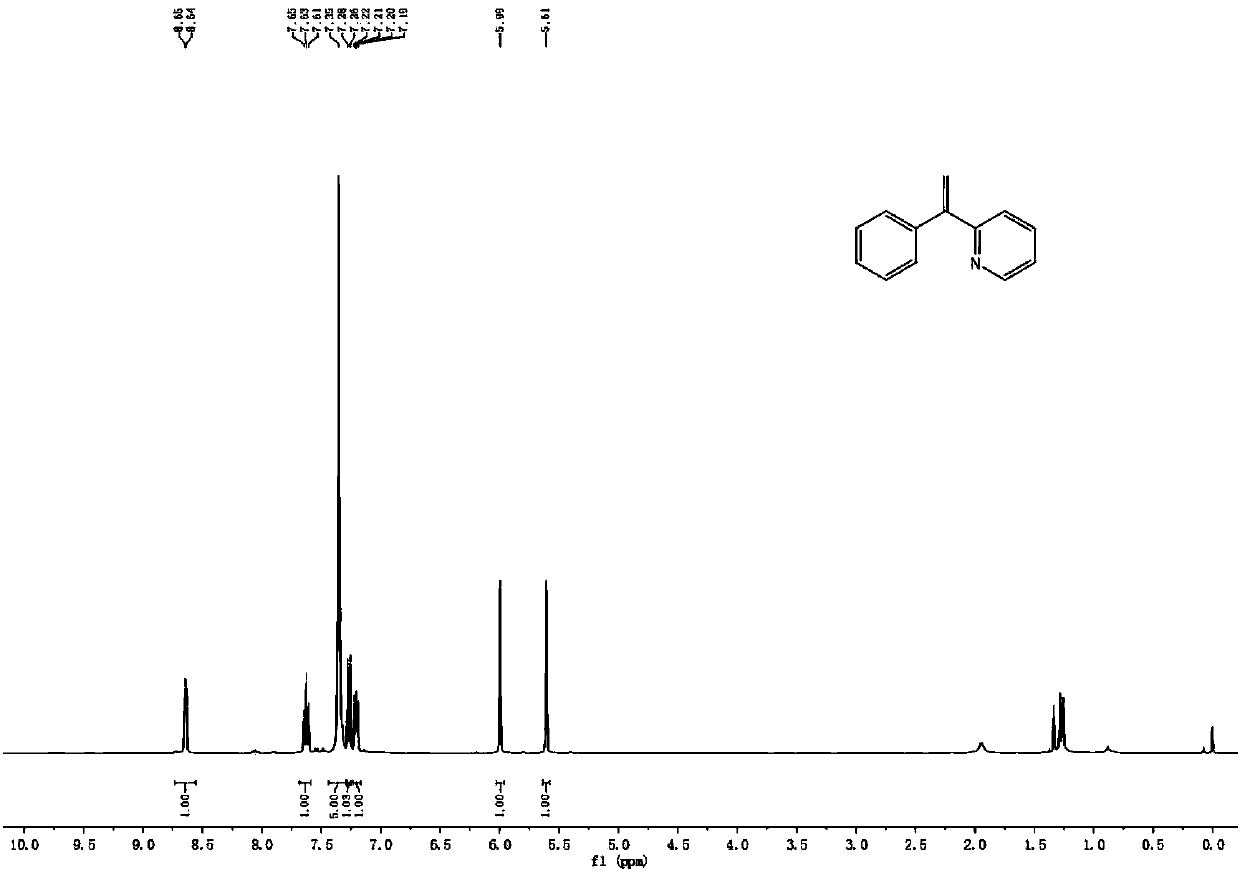
[0044] 2-Benzylpyridine (0.5mmol), sodium methoxide (1mmol), (NH 4 ) 2 S 2 o 8 (1mmol) and dimethyl sulfoxide (3mL) were added to the reactor, in a nitrogen environment, at a temperature of 120 ° C for 12h, after the reaction, the excess dimethyl sulfoxide was recovered by distillation, and the mixture was separated by chromatography , to obtain 2-(1-phenylethene)pyridine; yield 85%.
[0045] 1 H NMR (400MHz, CDCl 3)δ8.64(d, J=4.5Hz, 1H), 7.63(t, J=7.7Hz, 1H), 7.30-7.36(m, 5H), 7.27(d, J=8.6Hz, 1H), 7.21( dd, J=6.8, 5.5Hz, 1H), 5.99(s, 1H), 5.61(s, 1H).
[0046] 13 C NMR (101MHz, CDCl 3 )δ158.5, 149.4, 149.1, 140.4, 136.3, 128.4, 128.3, 127.8, 122.8, 122.4, 117.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com