Tissue-type plasminogen activator mutant and application thereof
A plasminogen and mutant technology, applied in the field of biomedicine, can solve the problems of reduced tPA drug titer, insufficient resistance, and no ability to enhance plasminogen activation, and achieve enhanced plasminogen activation. effect of ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
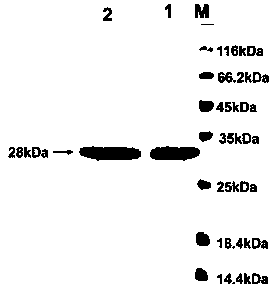
[0020] Construction, expression and purification of embodiment 1 tPA (A146Y) mutant
[0021] The tPA(A146Y) here is based on the hydrolase domain (tPA-SPD) of tPA for A146Y mutation, also referred to as tPA(A146Y), or tPA-SPD(A146Y) (the amino acid naming method is Chymotrypsinogen numbering):
[0022] (1) Construction of tPA-SPD-pPICZαA plasmid.
[0023] Using human hepatocyte cDNA as a template, the tPA-SPD gene fragment was amplified by PCR. Cut the tPA-SPD fragment with restriction endonuclease XhoI and SacI, and cut the pPICZαA plasmid (pPICZαA plasmid was purchased from Invitrogen) with the same endonuclease XhoI and SacI, and connect the tPA-SPD fragment to the pPICZαA plasmid with T4 ligase middle. The enzyme-linked product was transformed into Escherichia coli DH5α after heat stimulation at 42°C, plated, picked a single colony, and carried out gene sequencing. The DH5α strain containing the correct tPA-SPD sequence was expanded and cultivated, and the ZDNA plasmid s...
Embodiment 2
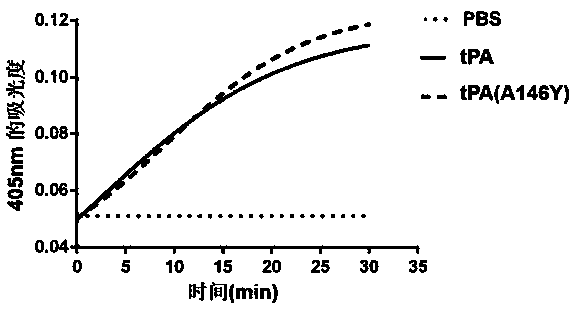
[0037] Example 2 Detection of tPA(A146Y) Mutant Enzyme Activity
[0038] Enzyme activity was determined by the reported chromogenic assay [Gorlatova NV (2003). Mapping of aconformational epitope on plasminogen activator inhibitor-1 by randommutagenesis. Implications for serpin function. J Biol Chem.]. The reaction principle is as follows, in a reaction system with a volume of 200 µL, a certain concentration of tPA protein is added. Then add the luminescent substrate S2288 (Chromogenix), tPA can specifically recognize the enzyme cutting site and cut off its chromophore-p-nitroaniline (pNA), and finally detect the absorbance value at 405nm by a microplate reader. The activity of the tPA enzyme can be assayed.
[0039] Specific measurement process:
[0040] (a) Materials
[0041] tPA, tPA(A146Y), obtained in Example 1 above, tPA substrate S-2288.
[0042] Buffer: 20mM Tris-HCl pH7.4, 150mM NaCl, 0.2% BSA. 0.22μm pore size membrane filter.
[0043] (b) steps
[0044] The mas...
Embodiment 3
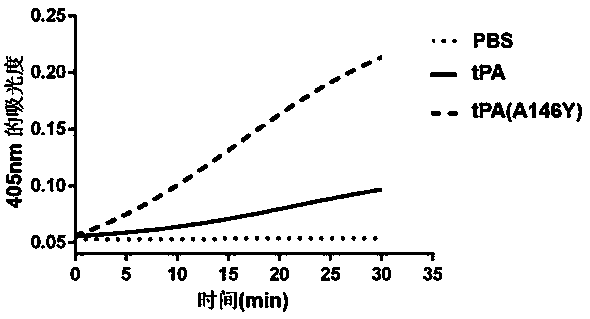
[0049] Example 3 Detection of tPA(A146Y) Activation Ability to Natural Substrate Plasminogen
[0050] Measured with the reported chromogenic assay [Gorlatova NV (2003). Mapping of aconformational epitope on plasminogen activator inhibitor-1 by randommutagenesis. Implications for serpin function. J Biol Chem.]. The reaction principle is as follows. In a reaction system with a volume of 200 µL, a certain concentration of tPA and plasminogen (PLG for short) are added, tPA activates PLG to generate plasmin (Pn for short), and then Pn-specific As for the luminescent substrate S-2403, Pn can specifically recognize the cleavage site and cut off its chromophore-p-nitroaniline (pNA), but tPA will not digest the luminescent substrate S2403. Finally, the activating ability of tPA to PLG can be determined by detecting the absorbance value at 405 nm with a microplate reader.
[0051] Specific measurement process:
[0052] (a) Materials
[0053] tPA obtained in Example 1 above, tPA (A146...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com