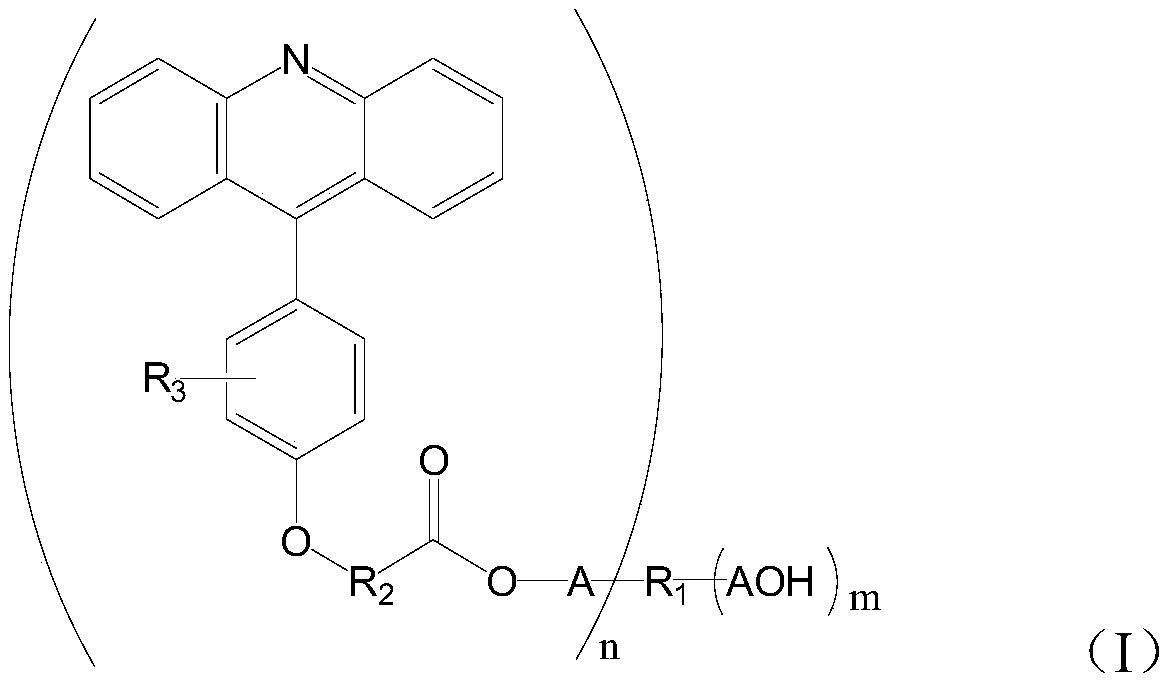
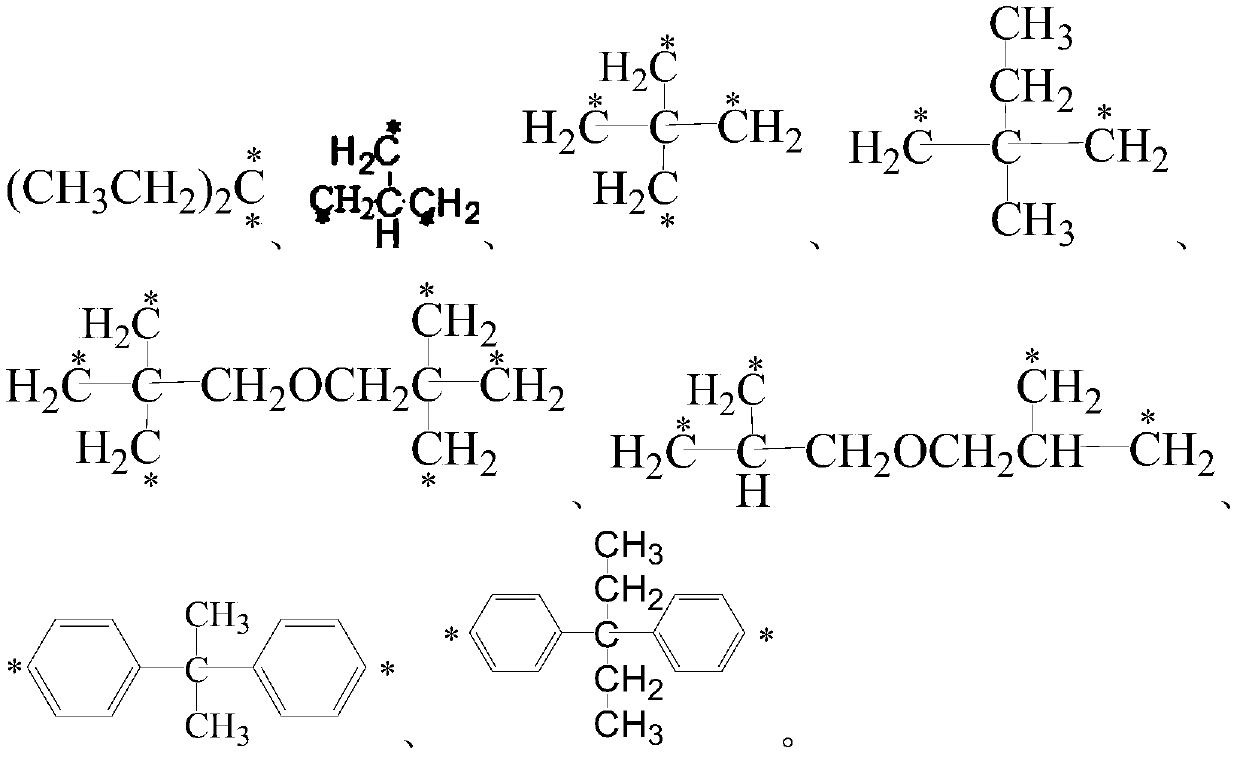
A kind of 9-phenylacridine macromolecular photosensitizer and its preparation method and application
A technology of phenylacridine and macromolecules, which is applied to 9-phenylacridine macromolecular photosensitizers and their preparation. The application of the photosensitizers in the field of photocuring can solve the problem of reducing product precision and affecting dryness. Film use, cumbersome process and other problems, to achieve the effect of reducing odor and toxicity, preventing yellowing and aging, and high photoactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
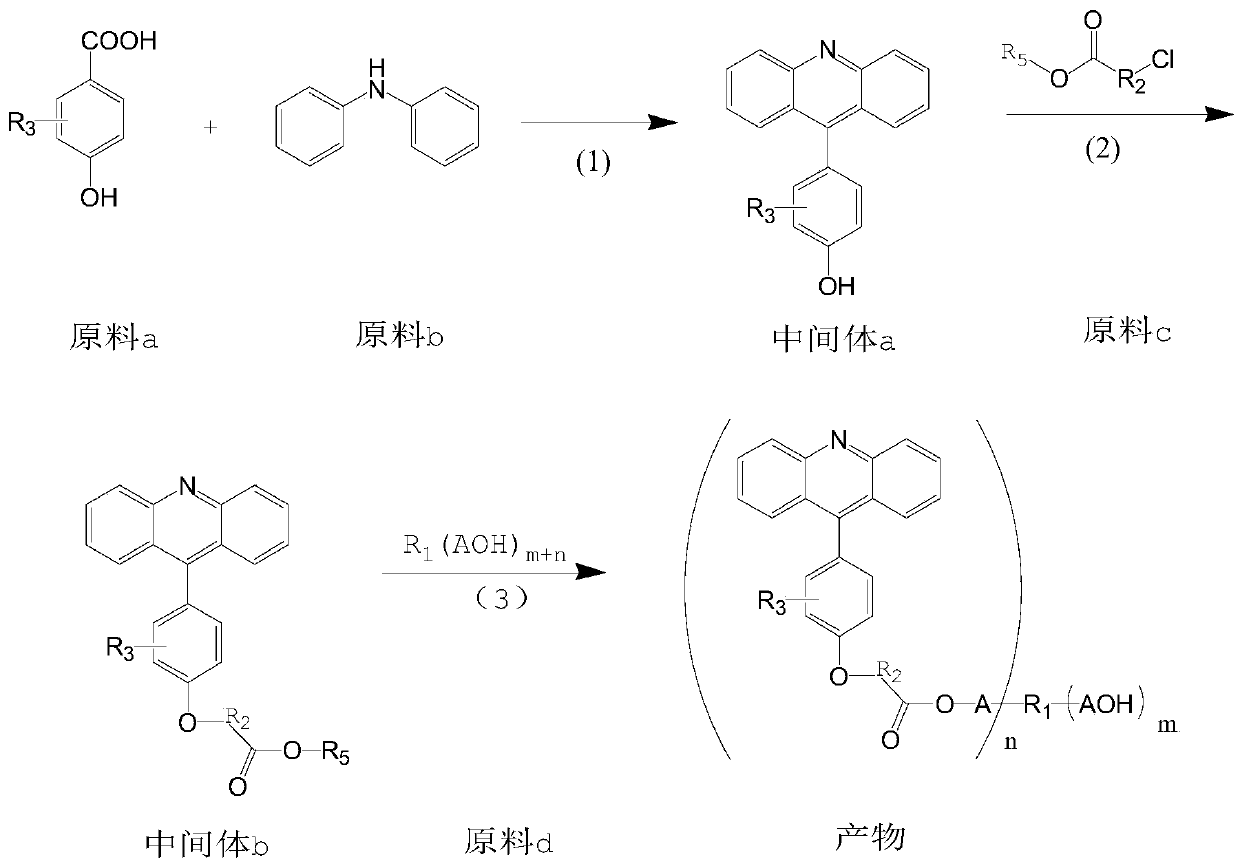
[0046] (1) Preparation of Intermediate 1a
[0047]
[0048] Add p-hydroxybenzoic acid 165.6g (1.2mol), diphenylamine 169.0g (1.0mol), zinc chloride 244.8g (1.8mol) and 85% phosphoric acid 115.3g (1.0mol) in the 1000ml four-necked bottle, stir and heat up to 200-210°C, react for 6h. Then cool down to 130-140°C, add 400g of 30% sulfuric acid dropwise, keep stirring at 80°C for 2h, add 300g of 80°C water and stir for 0.5h, let stand to separate the lower layer of water, then add 300g of water to repeat the above operation, and finally add to the reaction bottle 300g of ammonia water was added to the mixture, a large amount of orange solid was precipitated, suction filtered at room temperature, rinsed with methanol, and dried to obtain 257.4g of solid, namely intermediate 1a, with a purity of 98%;
[0049] The structure of intermediate 1a was confirmed by LCMS.
[0050] Mass spectrometry was used to obtain the 272 and 273 molecular fragment peaks with the help of the software...
Embodiment 2
[0063] (1) Preparation of Intermediate 2a
[0064]
[0065] Add 219.6g (1.2mol) of 4-hydroxyl-3-nitrobenzoic acid benzoic acid, 169.0g (1.0mol) of diphenylamine, 244.8g (1.8mol) of zinc chloride and 115.3g of 85% phosphoric acid in the 1000ml four-necked bottle (1.0mol), stir and heat up to 200-210°C, react for 6h, follow the reaction to the liquid phase. Cool down to 130-140°C, add 400g of 30% sulfuric acid dropwise, keep stirring at 80°C for 2 hours, add 300g of 80°C water and stir for 0.5h, let stand to remove the lower layer of water, then add 300g of water to repeat the above operation, and finally pour into the reaction bottle After adding 300g of ammonia water, a large amount of orange solid was precipitated, suction filtered, rinsed with methanol, and dried to obtain 300.2g of solid, namely intermediate 2a, with a purity of 98% and a yield of 95%;
[0066] The structure of intermediate 2a was confirmed by LCMS.
[0067] Mass spectrometry was used to obtain molecul...
Embodiment 3-9
[0079] Referring to the method of Example 1 or 2, the product 3-9 having the following structure was synthesized.
[0080] Product 3:
[0081]
[0082] Product 4:
[0083]
[0084] Product 5:
[0085]
[0086] Product 6:
[0087]
[0088] Product 7:
[0089]
[0090] Product 8:
[0091]
[0092] Product 9:
[0093]
[0094]
[0095] performance evaluation
[0096] The application performance of the photosensitizer of the present invention was evaluated by preparing an exemplary photocurable composition (ie, a photosensitive resin composition).
[0097] 1. Preparation of performance evaluation objects
[0098]
[0099] The photosensitive resin composition and propylene glycol monoethyl ether acetate having the composition shown in Table 1 were fully stirred and mixed, and coated on the surface of a 19 μm thick polyethylene terephthalate film as a support using a bar , evenly coated, and then dried in a dryer at 95° C. for 4 minutes to form a ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com