Detection method of genotoxic impurities in vilazodone hydrochloride
A technology of vilazodone hydrochloride and a detection method is applied in the field of detection of genotoxic impurity diamine and its hydrochloride in vilazodone hydrochloride, can solve the problems of difficulty in detection, low limit and the like, and achieves a good linear relationship, Realize the effect of effective control and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
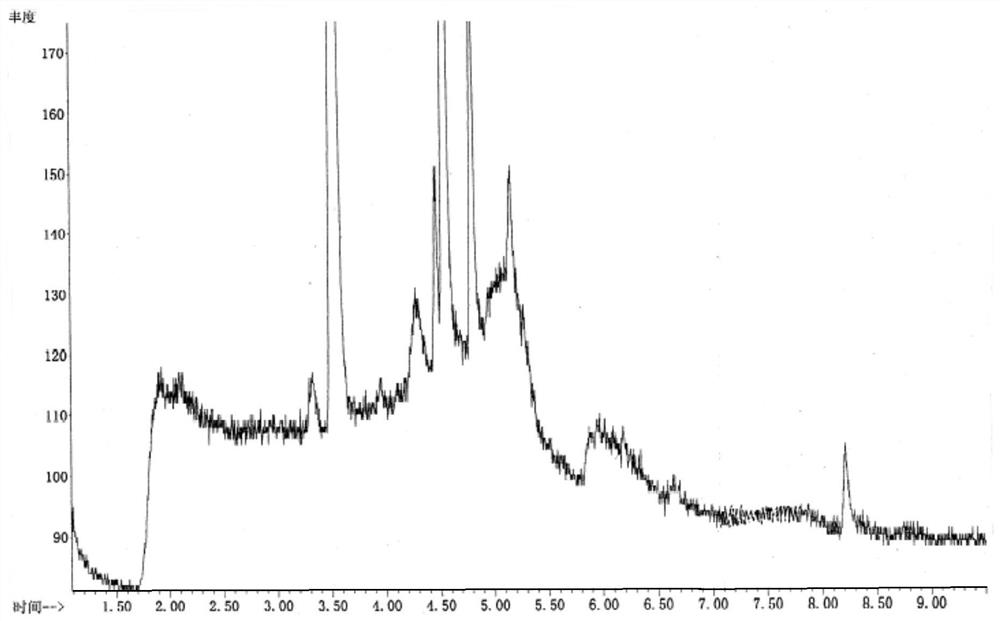
[0041] The localization test of embodiment 1 two (2-chloroethyl) amine hydrochloride
[0042]Gas phase conditions: use a cross-linked polyethylene glycol capillary column (DB-Wax, 30m×0.25mm×0.25μm), column temperature: 50°C, keep for 2min; heat up to 100°C at 20°C / min, keep for 5min; Gas: high-purity helium, carrier gas flow rate: 1.0ml / min, sampling method: split injection, split ratio: 5:1; purge carrier gas: nitrogen, purge temperature: 20°C time: 11min flow rate: 40ml / min, desorption temperature: 250°C time: 2min flow rate: 300ml / min, baking temperature: 280°C time: 2min flow rate: 200ml / min.
[0043] Mass spectrometry conditions: ionization source is EI, ion source temperature: 230°C, analyzer: single quadrupole mass analyzer, monitoring mode is single ion detection mode (SIM).
[0044] Blank solution: 0.1mol / L NaOH solution.
[0045] Reference substance stock solution configuration: Take 3.75mg of bis(2-chloroethyl)amine hydrochloride reference substance, weigh it ac...
Embodiment 2
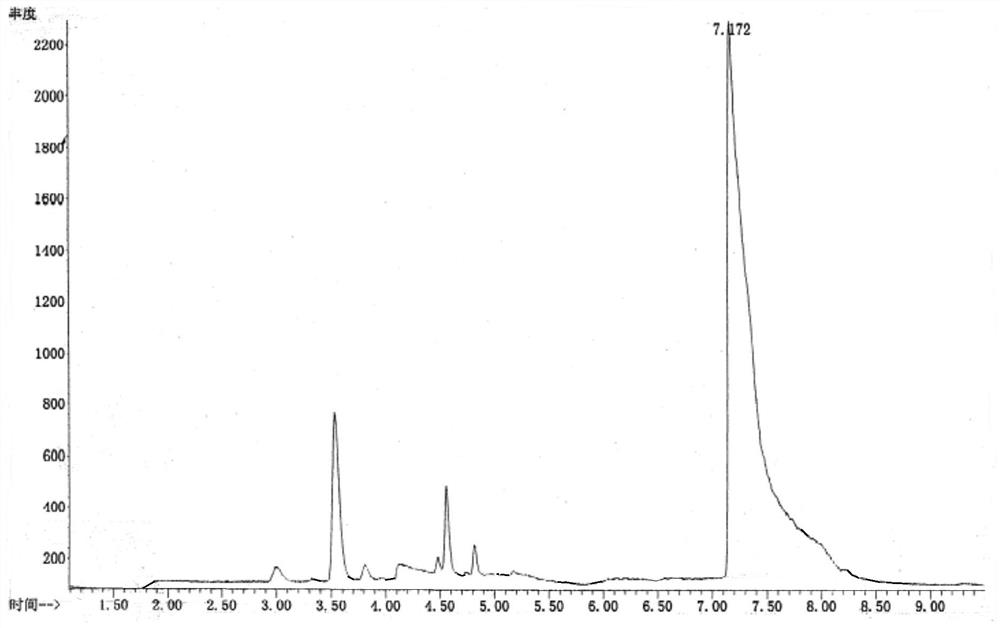
[0049] Embodiment 2 methodological investigation
[0050] Gas phase conditions: use a cross-linked polyethylene glycol capillary column (DB-Wax, 30m×0.25mm×0.25μm), column temperature: 50°C, keep for 2min; heat up to 100°C at 20°C / min, keep for 5min; Gas: high-purity helium, carrier gas flow rate: 1.0ml / min, sampling method: split injection, split ratio: 5:1; purge carrier gas: nitrogen, purge temperature: 20°C time: 11min flow rate: 40ml / min, desorption temperature: 250°C time: 2min flow rate: 300ml / min, baking temperature: 280°C time: 2min flow rate: 200ml / min. Mass spectrometry conditions: ionization source is EI, ion source temperature: 230°C, analyzer: single quadrupole mass analyzer, monitoring mode is single ion detection mode (SIM).
[0051] Reference substance solution: with reference to Example 1
[0052] 1. Linearity and range
[0053] Precisely measure 80 μl, 400 μl, 600 μl, 1.00ml, 1.60ml, 2ml of the stock solution of the reference substance solution, dissolve...
Embodiment 3
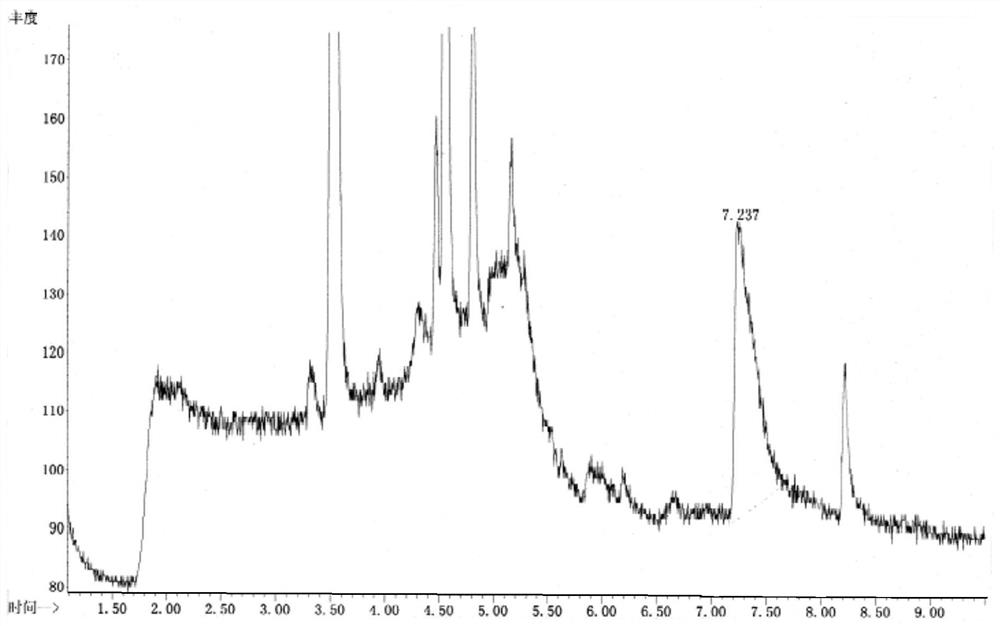
[0067] Example 3 Sample Determination
[0068] Gas phase conditions: use a cross-linked polyethylene glycol capillary column (DB-Wax, 30m×0.25mm×0.25μm), column temperature: 50°C, keep for 2min; heat up to 100°C at 20°C / min, keep for 5min; Gas: high-purity helium, carrier gas flow rate: 1.0ml / min, sampling method: split injection, split ratio: 5:1; purge carrier gas: nitrogen, purge temperature: 20°C time: 11min flow rate: 40ml / min, desorption temperature: 250°C time: 2min flow rate: 300ml / min, baking temperature: 280°C time: 2min flow rate: 200ml / min.
[0069] Mass spectrometry conditions: ionization source is EI, ion source temperature: 230°C, analyzer: single quadrupole mass analyzer, monitoring mode is single ion detection mode (SIM).
[0070] Solution preparation:
[0071] Please refer to Example 1 for the preparation of the reference solution.
[0072] Test solution: Accurately weigh 1 g of vilazodone hydrochloride, add 100 ml of 0.1 mol / L sodium hydroxide solution f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com