Simple synthesis method of aliphatic series hyperbranched polyester
A technology of hyperbranched polyester and synthesis method, applied in the field of synthesis of hyperbranched polyester, can solve the problems of limited application and development, slow degradation rate, etc., and achieves the effects of wide reaction temperature range, mild conditions and easy removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
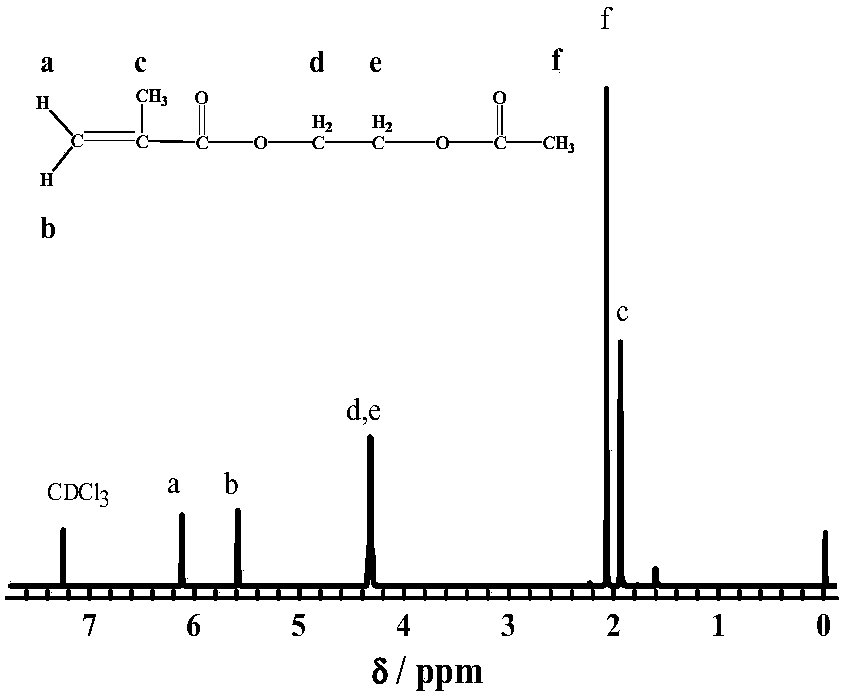
[0019] Into a 250mL three-necked flask were added 0.3mol of hydroxyethyl acrylate, 41mL of acetic anhydride, 85mL of tetrahydrofuran, and 0.3mol of sodium bicarbonate. React the reaction system at 30°C for 12 hours, filter out the solids, add an appropriate amount of dichloromethane, wash with saturated sodium bicarbonate solution, saturated sodium chloride solution, and water three times in sequence, remove water with anhydrous sodium sulfate, and obtain product. figure 1 It is the NMR diagram of the initiator monomer acetoxyethyl methacrylate. It can be seen from the figure that the peak position and integral area of each proton are consistent with the theoretical value. The purity of the synthesized monomer is 99% as measured by high performance liquid chromatography. %, indicating that the initiator monomer with higher purity was successfully synthesized.
Embodiment 2
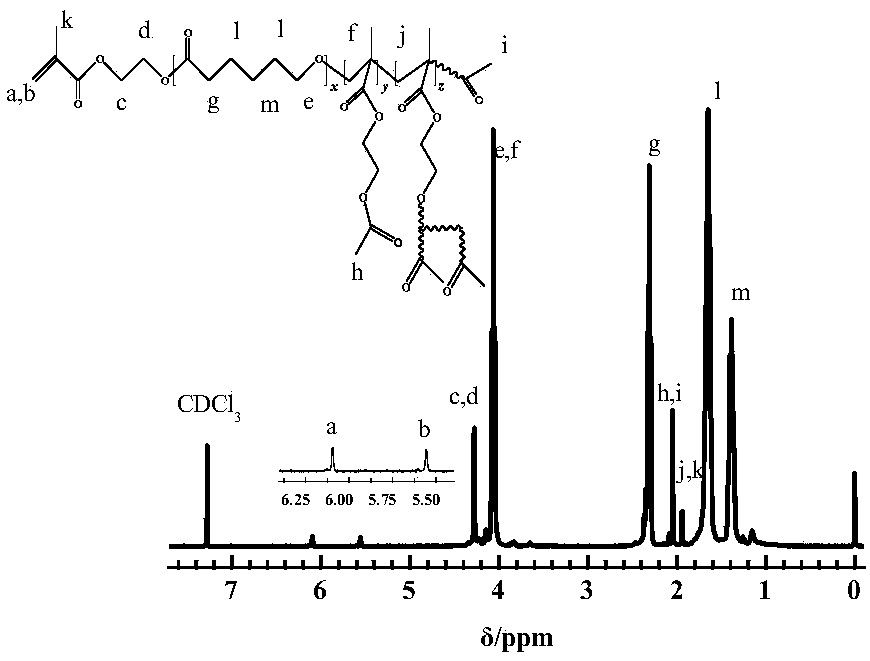
[0021] Under the protection of argon, 0.2mmol of acetoxyethyl methacrylate, 4mmol of caprolactone, and 0.2mL of toluene were respectively added to a 50mL reaction flask that had been baked repeatedly with a syringe. After freezing-pumping-filling with argon three times, the reaction flask was equilibrated at -30°C for 5 min. Then, under the protection of argon, add 0.2 mmol phosphazene base t-BuP 4 start reacting. After 5 hours, a small amount of benzoic acid was added to terminate the reaction, THF was added to dissolve, the phosphazene base was removed by acidic alumina filtration, n-hexane was settled, and the polymer was obtained by vacuum drying. It is measured that the conversion rate of AcEMA reaches 99.5%, and the conversion rate of caprolactone reaches 99.9%. The number-average molecular weight of gained polymkeric substance is 4900g / mol, and molecular weight distribution is 2.49, by the proton nuclear magnetic spectrogram of product ( figure 2 ) to calculate the ...
Embodiment 3
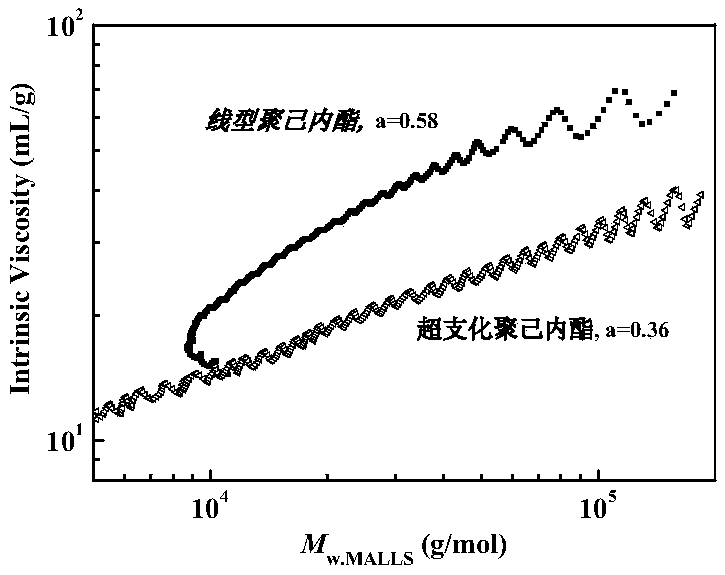
[0023] Under the protection of argon, 0.2mmol of acetoxyethyl methacrylate, 4mmol of succinic anhydride, and 0.2mL of toluene were respectively added into a 50mL reaction flask that had been baked repeatedly with a syringe. After freezing-pumping-filling with argon three times, the reaction flask was equilibrated at 60°C for 5 minutes. Then, under the protection of argon, add 0.2 mmol phosphazene base t-BuP 4 start reacting. After 1 hour, a small amount of benzoic acid was added to terminate the reaction, THF was added to dissolve, the phosphazene base was removed by acidic alumina filtration, n-hexane was settled, and the polymer was obtained by vacuum drying. It was measured that the conversion rate of AcEMA reached 99.6%, and the conversion rate of succinic anhydride reached 99.0%. The polymer obtained had a number average molecular weight of 6800 g / mol, a molecular weight distribution of 2.01 and a Mark-Howink constant of 0.45.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com