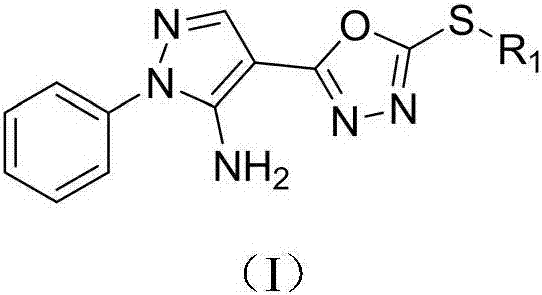
1-phenyl-5-amino-4-pyrazole bi-oxadiazole thioether compounds and application thereof
A technology of pyrazole-linked oxadiazole sulfide and compound, which is applied in the field of medicinal chemistry, can solve the problems of complex virus replication, infection and transmission mechanism, harm, aggravated accumulation of poison sources and large-scale diffusion, and achieve blunt Significant chemical effect, significant effect, good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: Preparation of 1-phenyl-4-((5-methylthio)-1,3,4-oxadiazolyl)-5-amino-1H-pyrazole (I1)
[0015] 1) Preparation of 1-phenyl-5-amino-1H-4-pyrazole hydrazide
[0016]
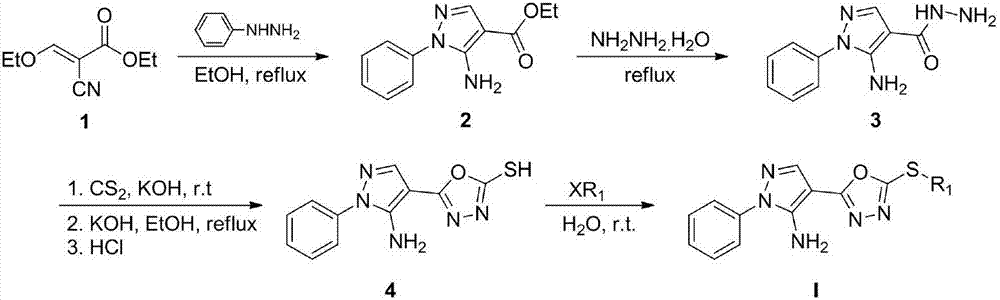
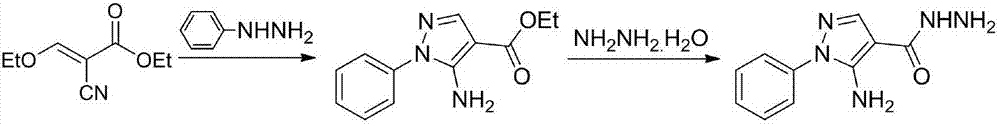
[0017] In a 250mL three-necked flask with a condenser and a thermometer, add ethyl ethoxymethylene acetate (62.1mmol), phenylhydrazine (93.2mmol), and ethanol (50mL) in sequence, heat to 80°C, react for 24h, and detect by TLC After the reaction was completed, lower it to normal temperature, distill off the solvent under reduced pressure, and dissolve the remaining solid in absolute ethanol (200mL) without purification, slowly add 80% hydrazine hydrate solution (100mmol) into the reaction solution, heat to reflux for 2 hours, and detect the reaction by TLC After the end, the system was lowered to normal temperature, suction filtered, the solid was washed with ethanol and dried to obtain the product, a white solid, with a yield of 70.5%.
[0018] 2) Preparation of 5-(5-amino-1-phenyl-1H-pyrazol-...
Embodiment 2
[0024] Embodiment 2: Preparation of 1-phenyl-4-((5-isopropyl)-1,3,4-oxadiazolyl)-5-amino-1H-pyrazole (I4):
[0025] Step (1) and (2) are with embodiment 1;
[0026] (3) Preparation of 1-phenyl-4-((5-isopropyl)-1,3,4-oxadiazolyl)-5-amino-1H-pyrazole (I4):
[0027]
[0028] In a 25mL reaction flask, add 5-(5-amino-1-phenyl-1H-pyrazol-4-yl)-1,3,4-oxadiazole-2-thiol (1.6mmol), Isobromopropane (1.6mmol) and 50% potassium hydroxide aqueous solution (20mL), stirred at room temperature, reacted for 24 hours, the reaction was detected by TLC, filtered to obtain a crude product, and column chromatography (dichloromethane / ethyl acetate=20 : 1) purification, target compound I4, white solid, yield 84.2%, melting point 88~89 ℃.
Embodiment 3
[0029] Embodiment 3: Preparation of 1-phenyl-4-((5-benzyl)-1,3,4-oxadiazolyl)-5-amino-1H-pyrazole (I28):
[0030] Step (1) and (2) are with embodiment 1;
[0031] (3) Preparation of 1-phenyl-4-((5-benzyl)-1,3,4-oxadiazolyl)-5-amino-1H-pyrazole (I28):
[0032]
[0033] In a 25mL reaction flask, add 5-(5-amino-1-phenyl-1H-pyrazol-4-yl)-1,3,4-oxadiazole-2-thiol (1.6mmol), Benzyl bromide (1.6mmol) and 50% potassium hydroxide aqueous solution (20mL), stirred at room temperature, reacted for 24 hours, the reaction was detected by TLC, filtered to obtain a crude product, and column chromatography (dichloromethane / ethyl acetate=20: 1) Purification, the target compound I28 is a white solid with a yield of 90.0% and a melting point of 164-165°C.
[0034] Through the similar synthetic method in embodiment 1, 2 and 3, the structure of the synthetic part 1-phenyl-5-amino-4-pyrazole bisoxadiazole sulfide compound and proton nuclear magnetic resonance spectrum, carbon spectrum and mass ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com