Stress inducible promoter, stress inducible promoter plant expression vector and expression method of induced target gene
A plant expression vector and target gene technology, applied in the field of stress-inducible promoters, can solve problems such as affecting plant physiological balance, protein overexpression, affecting yield, etc., to avoid energy waste and possible damage, efficient start, and enhance resistance. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] This example is about Brachypodium BdDREB-47 Cloning of gene promoters.
[0026] 1.1 Preparation of genomic DNA of Brachypodium
[0027] The leaves of normally growing Brachypodium bismuth were extracted by CTAB method, the concentration and quality of the extracted DNA were detected by ultra-trace nucleic acid protein analyzer Q5000, and the DNA concentration was diluted to 100ng / μl for use.
[0028] 1.2 Cloning of promoters
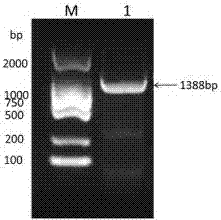
[0029] Using the above-extracted Brachypodium edodes DNA as a template, using the primers of SEQ ID NO: 2 and SEQ ID NO: 3, PCR amplification was performed to obtain the sequence of SEQ ID NO: 1.
[0030] The PCR reaction system is as follows:
[0031] reagent
Volume (μl)
ddH 2 O
5.8
2×Prime Star GC Buffer
10
dNTP mix (2.5mM)
2
Upstream primer (10 μM)
0.5
Downstream primer (10 μM)
0.5
Takara Prime Star Taq Enzyme
0.2
1
Overall system
2...
Embodiment 2
[0038] This example is about the construction of a stress-inducible promoter (pBdDREB-47) plant expression vector.
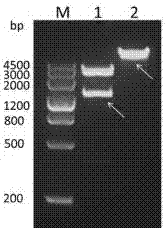
[0039] use Eco RI and Hin dⅢ (purchased from NEB Company) double-digested the recombinant plasmid pBdDREB-47-T and the vector pCAMBIA1381-GUS, see figure 2 , respectively recovered the target fragment (1388bp) and the pCAMBIA1381-GUS vector fragment, connected them with T4 DNA ligase (purchased from NEB Company), transferred them into E. coli competent cells Trans-T1 by heat shock method, and picked a single clone for positive See results after testing image 3 , amplify and extract the plasmid, and further digest and identify the positive plasmid ( EcoR I and Hin dⅢ double enzyme digestion) see the results Figure 4 , and the sequencing results are correct, indicating that the plant recombinant expression vector pC1381-pBdDREB-47-GUS was successfully constructed, and the pC1381-pBdDREB-47-GUS recombinant plasmid can be further transformed into Agrobac...
Embodiment 3
[0042] This example is about transforming tobacco with pC1381-pBdDREB-47-GUS recombinant plasmid through Agrobacterium-mediated method.
[0043] 3.1 Preparation of transgenic recipient material
[0044] Seeds of wild-type tobacco were sown in flower pots and placed in a 25-degree cultivation room (12h light / 12h dark), covered with plastic wrap to keep moisture, and the plastic wrap was gradually removed after the seedlings emerged. Two weeks later, the plants were transplanted, and when the tobacco was 2 months old, the leaves could be used for transgenic experiments.
[0045] 3.2 Disinfection and pre-cultivation of tobacco leaves
[0046] Tobacco leaves with good growth conditions were selected, and the tender leaves were cut off with scissors, and the tobacco leaves were soaked and sterilized with 75% ethanol for 50 seconds on the ultra-clean workbench. Then soak and sterilize with 2.5% sodium hypochlorite solution for 10 minutes, shake continuously during this period to f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com