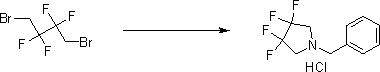
Synthesis method of 3,3,4,4-tetrafluoropyrrolidine
A technology of tetrafluoropyrrolidine and tetrafluoropyrrolidine hydrochloride, which is used in the synthesis of 3,3,4,4-tetrafluoropyrrolidine, and synthesizes 3,3,4,4-tetrafluoropyrrole from butanedione In the field of alkane, it can solve the problem of toxic yield of fluorine, and achieve the effect of clean reaction, simple operation and efficient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
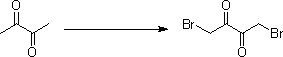
[0026] The first step, the preparation of 1,4-dibromobutanedione
[0027]
[0028] The reaction substrate butanedione (50 g, 0.58 mol) was added to chloroform (500 mL), and liquid bromine (186 g, 1.16 mol) was added dropwise at 20-30°C, then heated to 50°C and stirred for 3 hours. Cool down to 0°C, continue to stir for 0.5 hours, then filter, and wash the solid with water (1000 mL ) to obtain the intermediate 1,4-dibromobutanedione (55 g, 0.23 mol). Yield 39%.
[0029] 1 HNMR (CDCl 3 , 400MHz): δ 4.27(s, 4H).
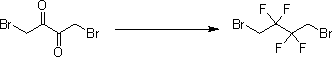
[0030] The second step, the preparation of 1,4-dibromo-2,2,3,3-tetrafluorobutane
[0031]
[0032] The intermediate 1,4-dibromobutanedione (15 g, 61.5 mmol) was cooled to -78°C, and sulfur tetrafluoride (30 g, 277.6 mmol) was introduced into it, then heated to 60°C and stirred for 3 hours. The reaction solution was dissolved in petroleum ether (100 mL), washed with saturated sodium bicarbonate (200 mL) and brine (100 mL) and spin-dried to obtain the intermed...
Embodiment 2
[0043] The first step, the preparation of 1,4-dibromobutanedione
[0044]
[0045]The reaction substrate diacetyl (50 g, 0.58 mol) was added to chloroform (500 mL), and liquid bromine (186 g, 1.16 mol) was added dropwise at 20-30°C, then heated to 60°C and stirred for 1 hour. Cool down to 0°C, continue to stir for 0.5 hours, then filter, and wash the solid with water (1000 mL ) to obtain the intermediate 1,4-dibromobutanedione (50 g, 0.21 mol). The yield is 35%.
[0046] 1 HNMR (CDCl 3 , 400MHz): δ 4.27(s, 4H).
[0047] The second step, the preparation of 1,4-dibromo-2,2,3,3-tetrafluorobutane
[0048]
[0049] The intermediate 1,4-dibromobutanedione (15 g, 61.5 mmol) was cooled to -78°C, and sulfur tetrafluoride (30 g, 277.6 mmol) was introduced into it, then heated to 80°C and stirred for 1 hour. The reaction solution was dissolved in petroleum ether (100 mL), washed with saturated sodium bicarbonate (200 mL) and brine (100 mL) and spin-dried to obtain the interme...
Embodiment 3
[0060] The first step, the preparation of 1,4-dibromobutanedione
[0061]
[0062] The reaction substrate diacetyl (50 g, 0.58 mol) was added to chloroform (500 mL), and liquid bromine (186 g, 1.16 mol) was added dropwise at 20-30°C, then heated to 70°C and stirred for 1 hour. Cool down to 0°C, continue to stir for 0.5 hours, then filter, and wash the solid with water (1000 mL ) to obtain the intermediate 1,4-dibromobutanedione (50 g, 0.21 mol). The yield is 35%.
[0063] 1 HNMR (CDCl 3 , 400MHz): δ 4.27(s, 4H).
[0064] The second step, the preparation of 1,4-dibromo-2,2,3,3-tetrafluorobutane
[0065]
[0066] The intermediate 1,4-dibromobutanedione (15 g, 61.5 mmol) was cooled to -78°C, and sulfur tetrafluoride (30 g, 277.6 mmol) was introduced into it, then heated to 70°C and stirred for 6 hours. The reaction solution was dissolved in petroleum ether (100 mL), washed with saturated sodium bicarbonate (200 mL) and brine (100 mL) and spin-dried to obtain the inter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com