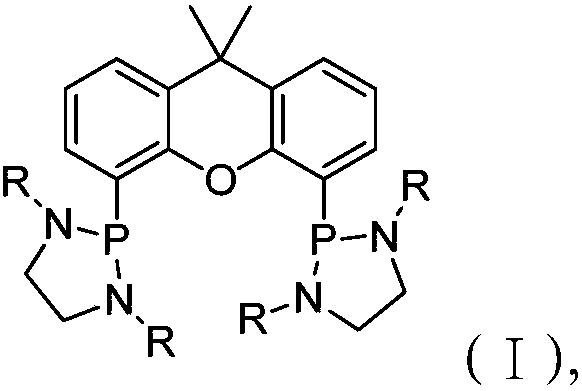
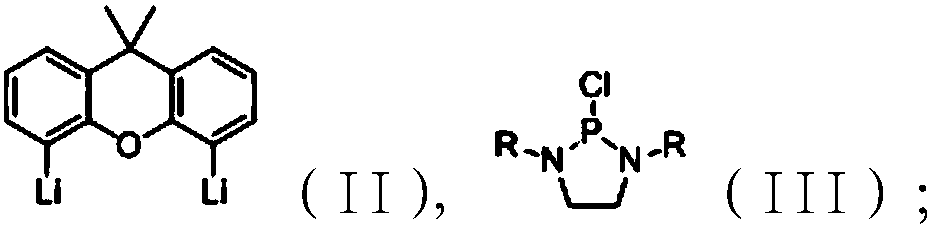Phosphoramidite ligand, catalyst and method for preparing 4-acetoxyl butaldehyde
A phosphoramidite and catalyst technology, which is applied in the field of preparation of 4-acetoxybutyraldehyde, can solve the problems of weak coordination, easy hydrolysis, and influence on catalyst separation and application, and achieve high reaction conversion rate and high selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] To prepare the phosphoramidite ligand L1, proceed as follows:
[0057] 1. Dissolve 9,9-dimethylxanthene (21.0g, 0.1mol) in 200mL of n-hexane. After it is completely dissolved, cool the system to -78°C in a dry ice acetone bath; Add n-BuLi (n-butyllithium) hexane solution (2M, 100mL) dropwise, remove the dry ice acetone bath, and naturally warm up to room temperature for 6 hours to obtain a lithium salt solution. After testing, the lithium salt has the formula the structural formula of (II);
[0058] 2. Dissolve phosphorus trichloride (27.5g, 0.2mol) and triethylamine (40.5g, 0.4mol) in 200mL of n-hexane, and place the solution in an ice-water bath to cool to 0-5°C; N, N-dimethylethylenediamine (17.6g, 0.2mol) n-hexane solution was slowly added dropwise to the mixture; after the dropwise addition, the ice-water bath was removed, the temperature was naturally raised to room temperature, and the reaction was continued for 4 hours; the reaction was completed, filtered, and...
Embodiment 2-6
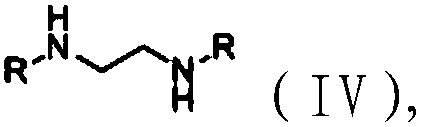
[0064] Compared with Example 1, Examples 2-6 only use ethylenediamine with different substituents as raw materials, and synthesize phosphoramidite ligands in the same way as Example 1, which are respectively numbered L2-L6.
[0065] The substituent ethylenediamine structural formula used in embodiment 2-6 is shown in formula (IV), wherein, the R groups in the compound of formula (IV) used in embodiment 2-6 are respectively isopropyl , Cyclohexyl, phenyl, p-methylphenyl, p-tert-butylphenyl.
[0066] The obtained product L2-L6 of embodiment 2-6 all has structural formula (I), wherein the R group in L2 is isopropyl group, and the R group in L3 is cyclohexyl group, and the R group in L4 is All are phenyl groups, the R groups in L5 are all p-methylphenyl groups, and the R groups in L6 are all p-tert-butylphenyl groups.
[0067] The phosphoramidite ligands L1-L6 prepared in Examples 1-6 were qualitatively analyzed by NMR, 1 H NMR (300MHz, CDCl 3 ) data looks like this:
[0068] ...
Embodiment 7
[0075] This embodiment is an embodiment of allyl acetate hydroformylation to synthesize 4-acetoxybutyraldehyde, and its preparation steps are as follows:
[0076] 10 mg of rhodium acetylacetonate was dissolved in 100 g of allyl acetate, 343.7 mg of ligand L1 was dissolved in 100 g of allyl acetate, the two solutions were added to a 1 L reaction kettle, and then 200 g of allyl acetate was added. Nitrogen replacement, synthesis gas replacement, filling with synthesis gas, boosting the pressure to 1MPa. Start the stirring, and raise the temperature to 100° C. to carry out the hydroformylation reaction. After the reaction was completed, the temperature was lowered to room temperature, and the pressure was released. The target product 4-acetoxybutyraldehyde was separated from the reaction liquid by rectification under reduced pressure, and the tower bottom liquid was reused as a catalyst solution. The raw material conversion rate is above 99%, and the yield of 4-acetoxybutyraldehy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com