A kind of modified graphite phase carbon nitride and its preparation method and application
A graphite phase carbon nitride and modification technology, applied in chemical instruments and methods, separation methods, nitrogen compounds, etc., can solve the problems of COS catalytic hydrolysis inactivity, catalytic activity, poor stability, specific surface area, low pore volume, etc. , to achieve the effect of increasing reactive sites, good performance and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
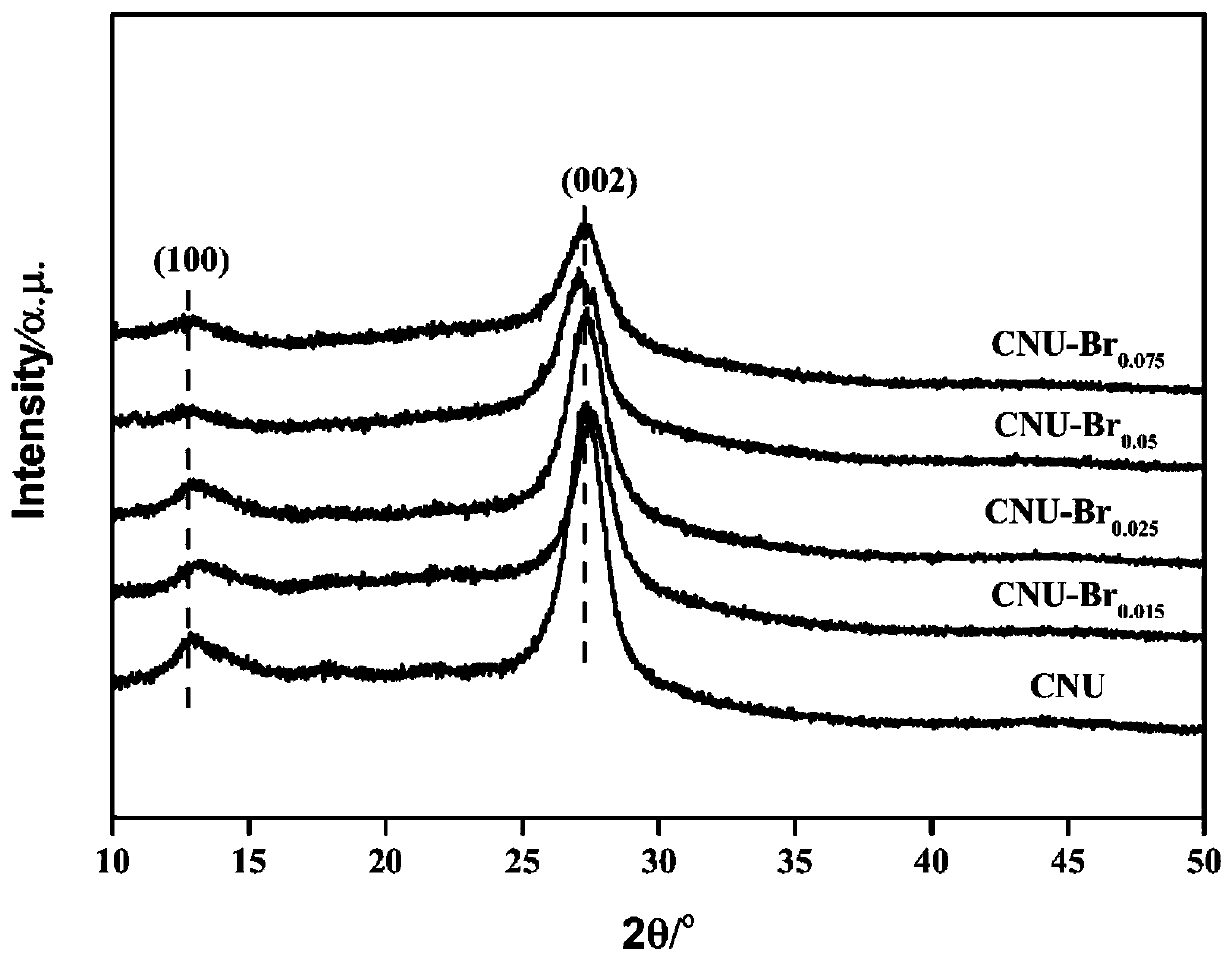
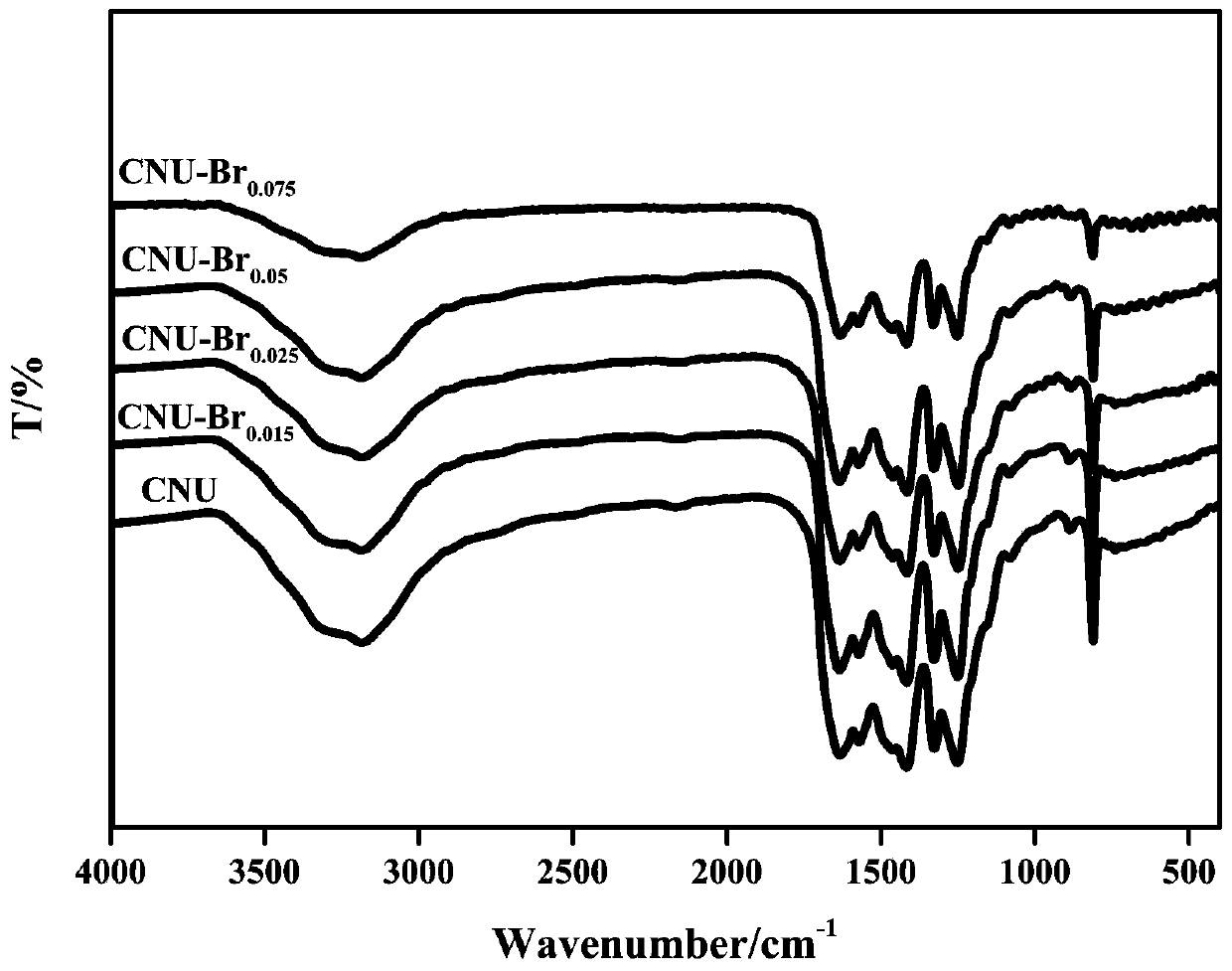
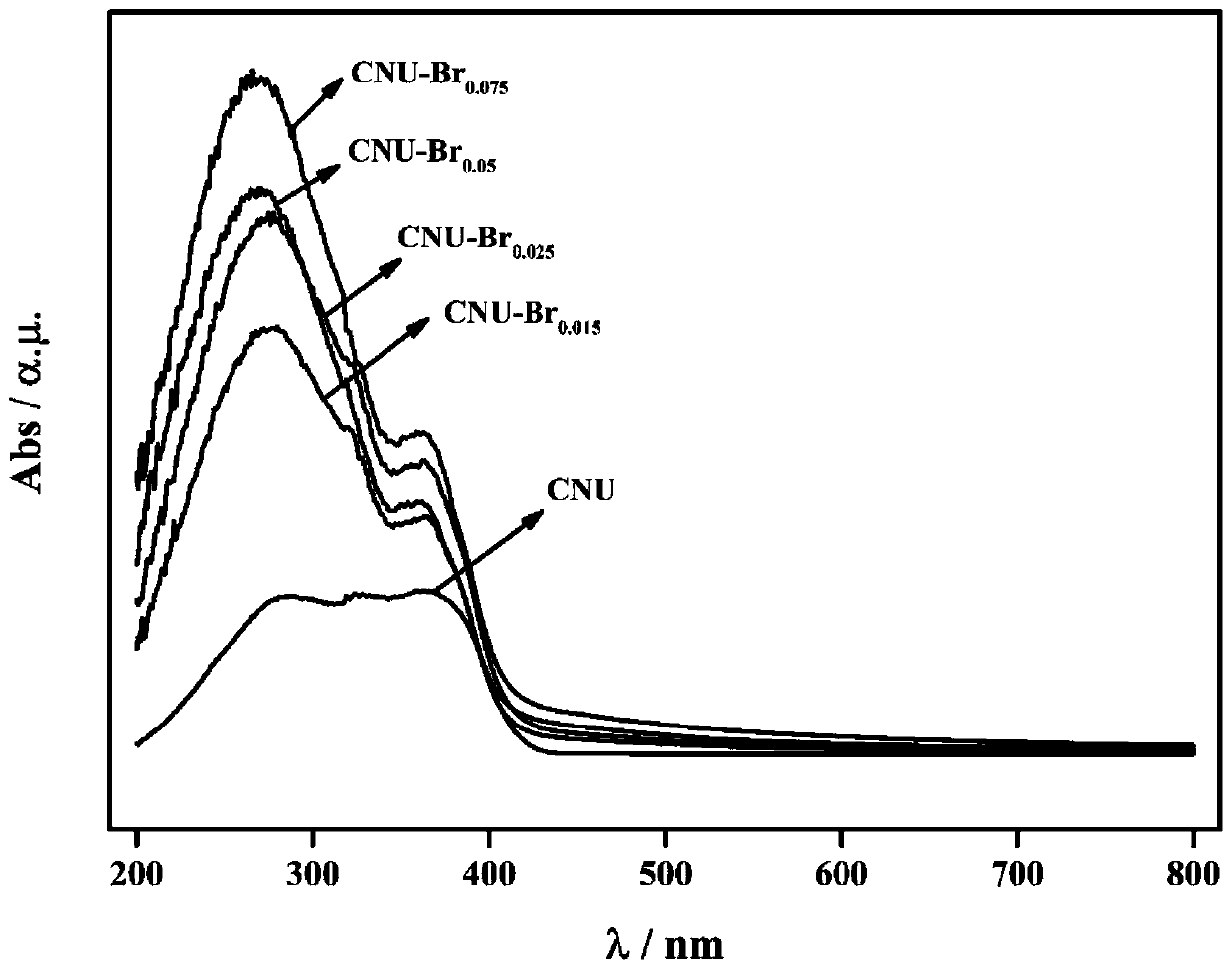
Image
Examples
Embodiment 1
[0041] Dissolve 10g of urea and 15mg of tetrabromobisphenol A in 100mL of water, stir and evaporate to dryness at 60°C. The evaporated sample was ground into a powder with a particle size of 250 mesh, placed in a heating device, and heated to 600°C at a rate of 5°C / min under a nitrogen atmosphere, and thermally polymerized for 2 hours. After natural cooling to room temperature, the synthesized samples were collected to obtain graphitic carbon nitride with nano-layered structure, which was named CNU-Br 0.015 .
Embodiment 2
[0043] Dissolve 10 g of urea and 25 mg of tetrabromobisphenol A in 100 mL of water, stir and evaporate to dryness at 90°C. The evaporated sample was ground into a powder with a particle size of 300 mesh, placed in a heating device, and heated to 500°C at a rate of 5°C / min under a nitrogen atmosphere, and thermally polymerized for 2 hours. After natural cooling to room temperature, the synthesized samples were collected to obtain graphitic carbon nitride with nano-layered structure, which was named CNU-Br 0.025 .
Embodiment 3
[0045] Dissolve 10g of urea and 50mg of tetrabromobisphenol A in 100mL of water, stir and evaporate to dryness at 80°C. The evaporated sample was ground into a powder with a particle size of 250 mesh, placed in a heating device, and heated to 550°C at a rate of 5°C / min under a nitrogen atmosphere, and thermally polymerized for 2 hours. After natural cooling to room temperature, the synthesized samples were collected to obtain graphitic carbon nitride with nano-layered structure, which was named CNU-Br 0.05 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com