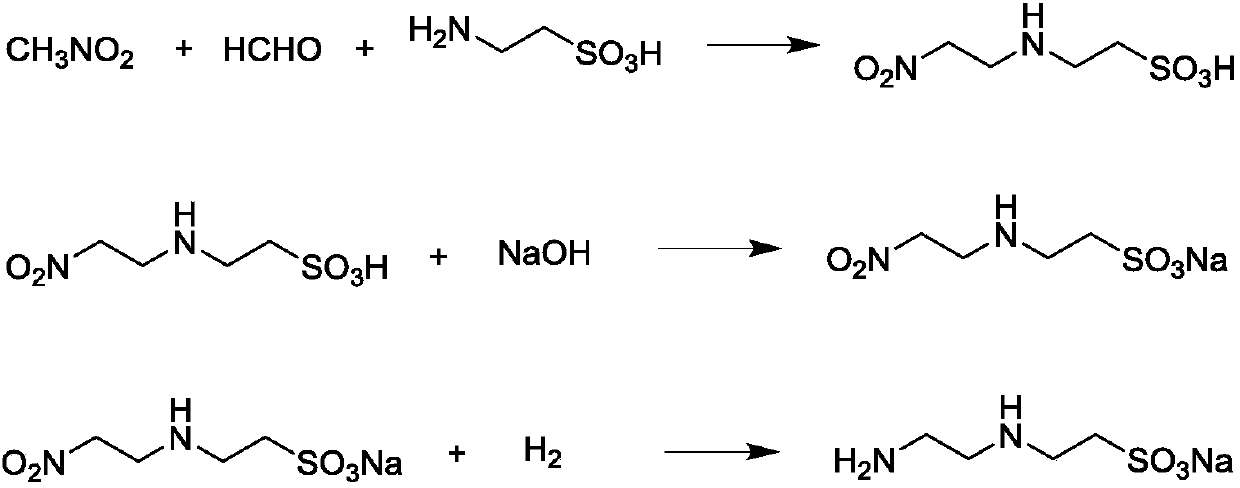
Method for preparing 2-((2-aminoethyl) amino)-ethanesulfonic acid monosodium salt
A technology of sodium ethylenediaminoethanesulfonate and sodium taurine, which is applied in the direction of sulfonic acid preparation, sulfonate preparation, organic chemistry, etc., and can solve the problem that sulfonic acid-type hydrophilic chain extenders do not have cost advantages and impurities Difficult to separate, high cost of raw materials and other problems, to achieve the effect of good industrialization prospects, high product selectivity, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) add 125 gram taurine, 118 gram 37wt% formaldehyde aqueous solution and 650 gram ethanol in the there-necked flask, stir 10 minutes under 25 ℃, in 30 minutes, 68 gram nitromethanes are added dropwise in the there-necked flask, be warming up to 30°C, stirred for 20 hours, the reaction solution was distilled under reduced pressure to remove light components, the solid was washed with 300 grams of water, and dried in vacuum at 50°C for 4 hours to obtain light yellow solid N-(2-nitroethyl)taurine 194.4 g, yield 98.0%, selectivity 99.8%. IR(KBr):ν=3340cm -1 (N-H), ν=1550cm -1 (NO 2 ), ν=1200cm -1 (SO 3 ); 1 H NMR (400 MHz, DMSO-D6), δ = 2.76 (t, 2H), δ = 3.01 (t, 2H), δ = 3.14 (t, 2H), δ = 4.69 (t, 2H).
[0040] (2) under stirring, slowly add 99 grams of N-(2-nitroethyl) taurine in 66.7 grams of 30wt% sodium hydroxide aqueous solution, temperature control 30 ℃, continue to stir for 10 minutes after the solid is added completely; Water was distilled off under pressu...
Embodiment 2
[0044] (1) Add 125 grams of taurine, 35 grams of paraformaldehyde and 2500 grams of methanol in a three-necked flask, stir at 25°C for 20 minutes, add 88 grams of nitromethane dropwise to the three-necked flask in 30 minutes, and heat up to 70 ℃, stirred for 4 hours, the reaction solution was distilled under reduced pressure to remove light components, the solid was washed with 260 grams of water, and dried in vacuum at 50 ℃ for 4 hours to obtain light yellow solid N-(2-nitroethyl) taurine 195.3 grams, yield 98.5%, selectivity 99.9%.
[0045](2) under stirring, slowly add 99 grams of N-(2-nitroethyl) taurine in 220 grams of 20wt% sodium bicarbonate aqueous solution, temperature control 40 ℃, continue to stir for 15 minutes after the solid is added completely; Water was distilled off under pressure to obtain 110 g of sodium N-(2-nitroethyl)taurate.
[0046] (3) 110 grams of N-(2-nitroethyl) sodium taurate, 7000 grams of ethanol, and 1 gram of Raney's nickel catalyst are added ...
Embodiment 3
[0049] (1) Add 125 grams of taurine, 95 grams of 37wt% formaldehyde solution and 1500 grams of dimethyl sulfoxide in a three-necked flask, stir at 25°C for 10 minutes, and add 76 grams of nitromethane dropwise to the three-necked flask within 30 minutes , heated to 55°C and stirred for 6 hours, the reaction solution was distilled under reduced pressure to remove light components, the solid was washed with 300 g of water, and dried under vacuum at 50°C for 4 hours to obtain a pale yellow solid N-(2-nitroethyl ) 194.9 grams of taurine, yield 98.2%, selectivity 99.8%.
[0050] (2) under stirring, slowly add 99 grams of N-(2-nitroethyl) taurine in the aqueous sodium hydroxide solution of 200 grams of 10wt%, temperature control 35 ℃, continue to stir for 10 minutes after solid is added completely; Water was distilled off under pressure to obtain 110 g of sodium N-(2-nitroethyl)taurate.
[0051] (3) 110 grams of N-(2-nitroethyl) sodium taurate, 3000 grams of N,N-dimethylformamide, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com