Dual-targeting delivery method of pectin nanoparticles modified by folic acid
A nanoparticle, dual-targeting technology, applied in the fields of advanced nanotechnology, biopharmaceuticals, and polymer materials, can solve problems such as indigestibility, avoid sudden release, improve stability and yield, and improve biocompatibility sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
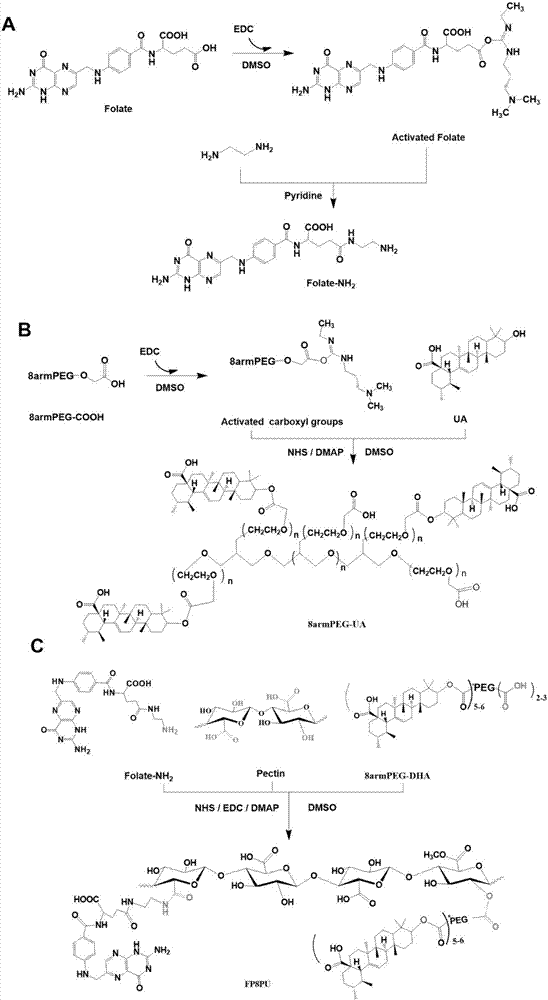
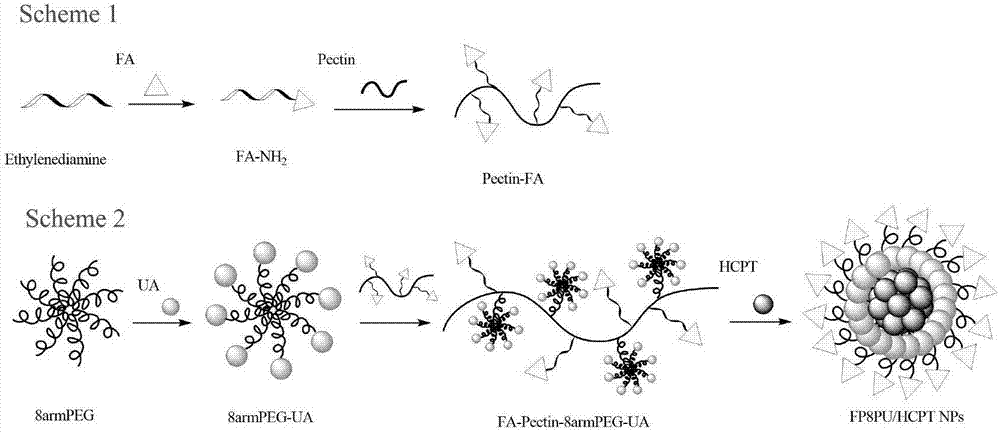
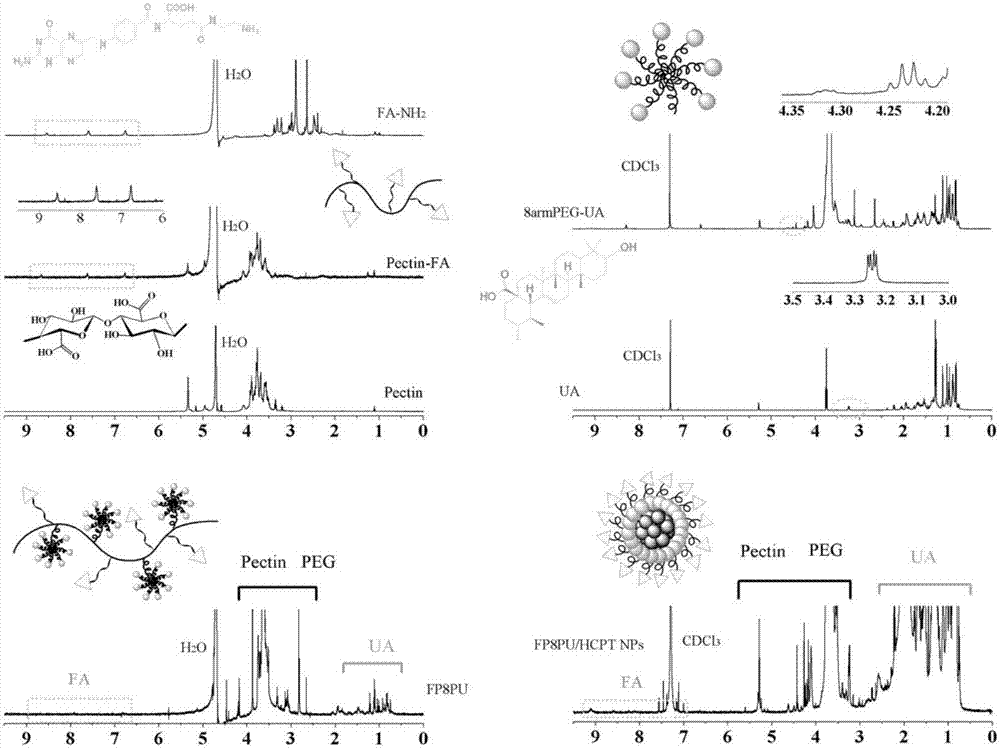
[0034] (1) Dissolve 1.0 g of folic acid in dimethyl sulfoxide, add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) to activate the terminal carboxyl group of folic acid, and react After 30 minutes, add ethylenediamine and pyridine, stir evenly, and react in the dark for 24 hours to obtain aminofolate (FA-NH 2 ), transfer the reaction solution to the dialysis membrane, use phosphate buffer solution (pH=7.4) as the outer fluid for dialysis, dialyze overnight, change the outer fluid for dialysis every 4 hours, collect the solution in the dialysis membrane, and freeze-dry to obtain a yellow powder;
[0035] (2) Dissolve 1.0g pectin in deionized water, stir well, add EDC to react for 30 minutes, activate the carboxyl group of pectin, then add 0.1g FA-NH 2 React with 4-dimethylaminopyridine (DMAP) in the dark for 24 hours, transfer the reaction solution to the dialysis membrane, use phosphate buffer solution (pH=7.4) as the external fluid for dialysis, dialyze over...
Embodiment 2
[0041] (1) Dissolve 1.0 g of folic acid in dimethyl sulfoxide, add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) to activate the terminal carboxyl group of folic acid, and react After 30 minutes, add ethylenediamine and pyridine, stir well, and react in the dark for 24 hours to obtain aminated folic acid (FA-NH2), transfer the reaction solution to the dialysis membrane, and use phosphate buffer solution (pH=7.4) as the external fluid for dialysis , dialyze overnight, change the outer dialyzed fluid every 4 hours, collect the inner solution of the dialyzed membrane, freeze-dry to obtain a yellow powder;
[0042] (2) Dissolve 1.0g of pectin in deionized water, stir well, add EDC to react for 30 minutes, activate the carboxyl group of pectin, then add 0.2g of FA-NH2 and 4-dimethylaminopyridine (DMAP) to avoid light reaction After 24 hours, the reaction solution was transferred to the dialysis membrane, and the phosphate buffer solution (pH=7.4) was used as the...
Embodiment 3
[0048] (1) Dissolve 1.0 g of folic acid in dimethyl sulfoxide, add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) to activate the terminal carboxyl group of folic acid, and react After 30 minutes, add ethylenediamine and pyridine, stir well, and react in the dark for 24 hours to obtain aminated folic acid (FA-NH2), transfer the reaction solution to the dialysis membrane, and use phosphate buffer solution (pH=7.4) as the external fluid for dialysis , dialyze overnight, change the outer dialyzed fluid every 4 hours, collect the inner solution of the dialyzed membrane, freeze-dry to obtain a yellow powder;
[0049] (2) Dissolve 1.0g of pectin in deionized water, stir well, add EDC to react for 30 minutes, activate the carboxyl group of pectin, then add 0.1g of FA-NH2 and 4-dimethylaminopyridine (DMAP) to avoid light reaction After 24 hours, the reaction solution was transferred to the dialysis membrane, and the phosphate buffer solution (pH=7.4) was used as the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com