Preparation method of novel sepsis animal model
An animal model and sepsis technology, applied in the field of preparation of animal models of sepsis, can solve problems such as deviation from clinical practice, and achieve the effects of small human influence, simple preparation method and clear genetic information
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] see Figure 1-3 , the present invention provides a kind of technical scheme: a kind of preparation method of new sepsis animal model, adopts SPF level 8 weeks old Balb / c mouse, body weight 25-30g, after adaptive feeding 1 week, carry out experiment, its It is characterized in that it comprises the following steps;
[0030] Step 1: In the stage of adaptive feeding and modeling, the mice were free to eat and drink, and the light / dark alternate lighting was adopted at intervals of 12 hours. The serial number corresponding to each mouse is fixed and unique. Using the random number table method, they were equally divided into two large groups, female and male, and each large group was further divided into A (n=6), B (n=8), C (n=8), D (n= 8) 4 teams;
[0031] Step 2: Group A: control group, at each injection time point, give the same volume of normal saline as the drug and (or) bacteria;
[0032] Step 3: Group B: Cyclosporin A injection group: on day -5, -3, and -1, weigh...
Embodiment 1
[0039] 1. Experimental animals and feeding
[0040] SPF grade 8-week-old Balb / c mice weighing 25-30 g were used. Experimental animals were provided by the Experimental Animal Center of the Academy of Military Medical Sciences, license number: SCXK (Beijing) 2014-0013. Animals were fed adaptively for 1 week before the experiment. In the stage of adaptive feeding and model establishment, the mice had free access to food and water, and were illuminated alternately with light / dark at intervals of 12 hours, and the temperature and humidity were suitable.
[0041] 2. Experiment with other main materials
[0042] Cyclosporine A (MCE Company), Escherichia coli DH5α (preserved in our laboratory), normal saline for injection (Chenxin Pharmaceutical Co., Ltd.), disposable syringe (Shengguang Medical Products Co., Ltd.), tryptone (Oxoid Company ), yeast powder (Oxoid), sodium chloride (Sinopharm Chemical Reagent Beijing Co., Ltd.).
[0043] 3. Preparation method:
[0044] 3.1 On the ...
Embodiment 2
[0053] 1. Specimen collection
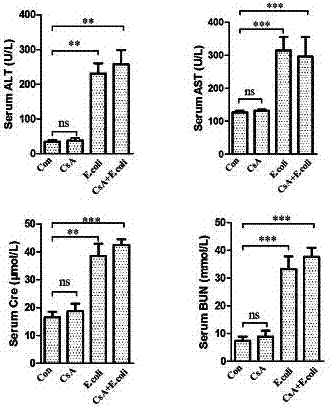
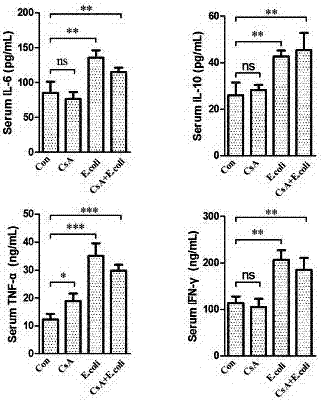
[0054] Both male and female groups were divided into four subgroups. Each subgroup was injected with bacteria or normal saline on the 0th day, starting 12 hours after the injection, and observed the living conditions of the animals. The abdominal body temperature of the mice became lower and the hair stood upright. 1. When there is no response to touch, give 5mg / kg chloral hydrate deep anesthesia, draw blood from the heart, and add part of the whole blood to a sterile collection tube with 50 μL of sterile heparin sodium injection in advance for blood bacterial culture , the other part of whole blood was added to a 1.5mL collection tube, placed at room temperature for 2 hours, centrifuged at 8000rpm for 5 minutes, and the serum was separated for the detection of serological indicators of tissue damage. Kidney, lung, spleen and other tissues, take the left lobe of liver, left kidney, and left lung, and try to ensure that the parts of the samples a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com