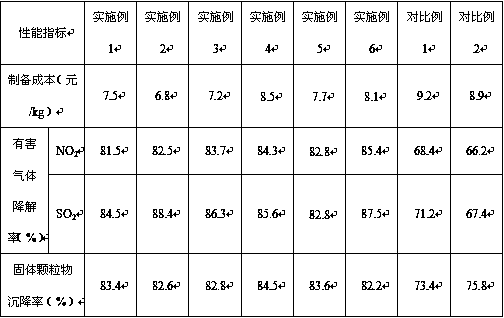
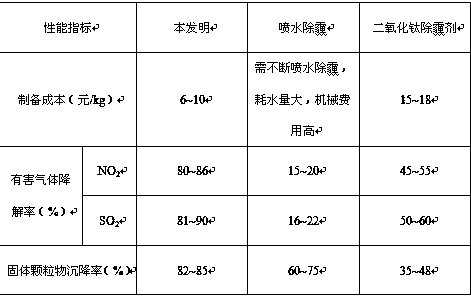
Low-cost haze-removing auxiliary agent capable of rapidly eliminating haze and preparation method
A technology for eliminating smog and haze additives, applied in separation methods, chemical instruments and methods, gas treatment, etc., can solve problems such as high cost, large water consumption, and poor haze removal effect, and achieve cost control, efficient decomposition, The effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation process is:
[0038](1) Add 8 parts by weight of ferric nitrate hydrate and 22 parts by weight of ethylene glycol methyl ether to 68 parts by weight of water and mix evenly, add 2 parts by weight of tetrabutyl titanate dropwise in a constant temperature water bath at 60°C, and react with magnetic stirring for 80 minutes , to prepare ferric oxide nanocrystalline sol; the speed of magnetic stirring is 130r / min;
[0039] (2) Add 0.8 parts by weight of zinc acetate to 58 parts by weight of absolute ethanol, stir magnetically at 50°C until fully dissolved to obtain a zinc oxide precursor solution, add 41.2 parts by weight of lithium hydroxide solution dropwise, and stir vigorously for 50 minutes , filtered, cleaned, and dried to obtain zinc oxide quantum dots; the speed of magnetic stirring is 130r / min; the stirring speed of vigorous stirring is 700r / min; the lithium hydroxide solution is a lithium hydroxide solution with a mass concentration of 0.3%;
[004...
Embodiment 2
[0046] The preparation process is:
[0047] (1) Add 6 parts by weight of ferric nitrate hydrate and 18 parts by weight of ethylene glycol methyl ether to 75 parts by weight of water and mix evenly, add 1 part by weight of tetrabutyl titanate dropwise in a constant temperature water bath at 55°C, and react with magnetic stirring for 100 minutes , to prepare ferric oxide nanocrystalline sol; the speed of magnetic stirring is 100r / min;
[0048] (2) Add 0.5 parts by weight of zinc acetate to 55 parts by weight of absolute ethanol, stir magnetically at 40°C until fully dissolved to obtain a zinc oxide precursor solution, add 44.5 parts by weight of lithium hydroxide solution drop by drop, and stir vigorously for 40 minutes , filtered, cleaned, and dried to obtain zinc oxide quantum dots; the speed of magnetic stirring is 100r / min; the stirring speed of vigorous stirring is 600r / min; the lithium hydroxide solution is a lithium hydroxide solution with a mass concentration of 0.2%;
...
Embodiment 3
[0055] The preparation process is:
[0056] (1) Add 10 parts by weight of ferric nitrate hydrate and 25 parts by weight of ethylene glycol methyl ether into 62 parts by weight of water and mix evenly, add 3 parts by weight of tetrabutyl titanate dropwise in a constant temperature water bath at 65°C, and react with magnetic stirring for 100 minutes , to prepare ferric oxide nanocrystalline sol; the speed of magnetic stirring is 150r / min;
[0057] (2) Add 1 part by weight of zinc acetate to 60 parts by weight of absolute ethanol, stir magnetically at 60°C until fully dissolved to obtain a zinc oxide precursor solution, add 39 parts by weight of lithium hydroxide solution drop by drop, and stir vigorously for 60 minutes , filtered, cleaned, and dried to obtain zinc oxide quantum dots; the speed of magnetic stirring is 150r / min; the stirring speed of vigorous stirring is 800r / min; the lithium hydroxide solution is a lithium hydroxide solution with a mass concentration of 0.5%;
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com