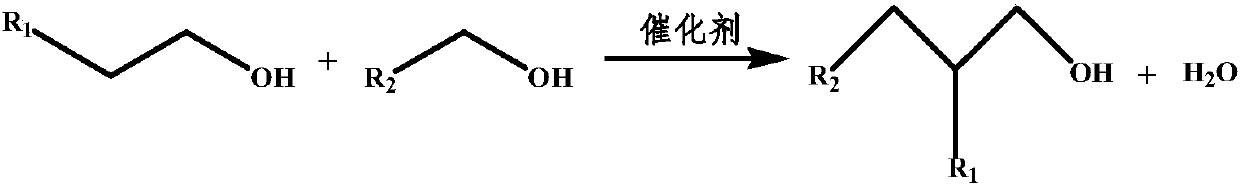
Compound higher alcohol catalyst, preparation method and application
A technology for catalysts and higher alcohols, applied in catalyst activation/preparation, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve the problems of decreased catalytic activity and increased reaction energy consumption, and achieves low production cost, green process, and improved catalytic performance. The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A) Meso-Al 2 o 3 - Preparation of MgO
[0036] 1.89 g of CTAB was dissolved in 35 ml of deionized water to obtain a solution A with a CTAB concentration of 0.15 mol / L. 64.1gMg(NO 3 ) 2 .6H 2 O and 3.68g Al(NO 3 ) 3 .9H 2 O was dissolved in 52 ml deionized water to obtain Mg 2+ and Al 3+ The total concentration is 5.0mol / L solution B. Under the condition of stirring, solution B is added dropwise in solution A to obtain solution C, and the molar composition of C solution is:
[0037] (Mg 2+ +Al 3+ ):CTAB:H 2O=1:0.02:25. Mix 100ml ammonia water and 300ml deionized water to get NH 4 + D solution with a concentration of 3.45mol / L. Control the temperature of solution C at 50°C, add solution D dropwise to solution C under stirring conditions, control the pH value of the system to 9.5, stir for 2 hours under this condition, and dynamically crystallize the resulting slurry under hydrothermal conditions at 100°C After 16 hours of crystallization, the obtained so...
Embodiment 2
[0043] A) Meso-ZrO 2 - Preparation of MgO
[0044] 4.67 g of CTAB was dissolved in 44 ml of deionized water to obtain solution A with a CTAB concentration of 0.29 mol / L. 64.10g Mg(NO 3 ) 2 .6H 2 O and 2.79g Zr(NO 3 ) 4 .5H 2 O was dissolved in 66 ml deionized water to obtain Mg 2+ and Zr 4+ Solution B with a total concentration of 3.9 mol / . Under the condition of stirring, solution B is added dropwise in solution A to obtain solution C, and the molar composition of C solution is:
[0045] (Mg 2+ +Zr 4+ ):CTAB:H 2 O=1:0.05:30. . Mix 100ml ammonia water and 300ml deionized water to get NH 4 + Solution D with a concentration of 3.45 mol / L. Control the temperature of solution C at 40°C, add solution D dropwise to solution C under stirring conditions, control the pH value of the system to 10.0, stir for 2 hours under this condition, and dynamically crystallize the resulting slurry under hydrothermal conditions at 130°C 12h, after the crystallization was completed,...
Embodiment 3
[0052] A) Meso-MgO-CeO 2 preparation of
[0053] 9.11 g of CTAB was dissolved in 133 ml of deionized water to obtain a solution A with a CTAB concentration of 0.19 mol / L. 64.10gMg(NO 3 ) 2 .6H 2 O and 2.52g Ce(NO 3 ) 3 ·6H 2 O was dissolved in 200ml deionized water to obtain Mg 2+ and Ce 3+ Solution B with a total concentration of 1.3 mol / . Under the condition of stirring, solution B is added dropwise in solution A to obtain solution C, and the molar composition of C solution is:
[0054] (Mg 2+ +Ce 3+ ):CTAB:H 2 O=1:0.1:80. Mix 100ml ammonia water and 590ml deionized water to get NH 4+ Solution D with a concentration of 2.00 mol / L. Control the temperature of solution C to 70°C, add solution D dropwise to solution C under stirring conditions, control the pH value of the system to 9.0, stir under this condition for 1 hour, and dynamically crystallize the resulting slurry under hydrothermal conditions at 140°C After 10 hours of crystallization, the obtained solid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com