A hydrotalcite-derived cobalt-based catalyst is promoted by an auxiliary for autothermal reforming of acetic acid for hydrogen production
A cobalt-based catalyst and autothermal reforming technology, applied in metal/metal oxide/metal hydroxide catalysts, catalyst activation/preparation, physical/chemical process catalysts, etc., can solve problems such as catalyst deactivation and carbon deposition , to achieve high activity, promote diffusion, and good catalytic stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
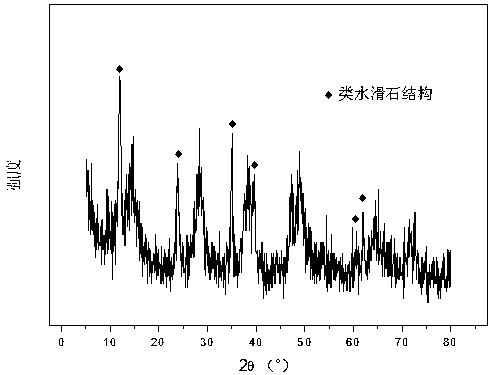
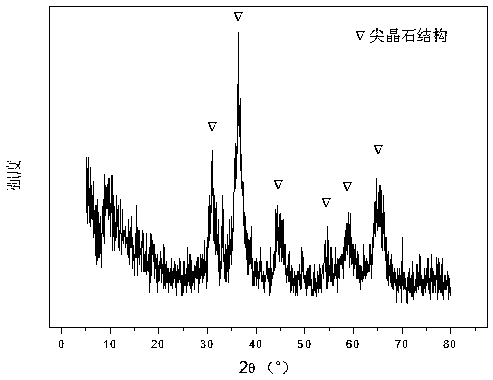
[0026] Weigh 2.329g Co(NO 3 ) 2 ·6H 2 O, add 74.7 ml of deionized water to make solution #1. Weigh out 23.904g NaOH and 3.950g Na 2 CO 3 , and 634.9 ml of deionized water was added to prepare precipitant solution #2. Under the conditions of 68°C and PH=10.5±0.5, the solutions #1 and #2 were subjected to co-precipitation reaction, and the stirring was continued for 18 hours; after the aging, the mixed solution was filtered and washed three times, and the resulting precipitate was transferred to vacuum drying In the oven, drying at 105 °C for 12 hours, the hydrotalcite-like precursor was obtained, and its typical X-ray diffraction spectrum is shown in the attached figure 1 then calcined at 650°C for 4 hours, tableted and sieved to obtain a 20-40 mesh CDUT-CAF-0 catalyst, the composition of which is (Co 3 O 4 ) 0.12 (Al 2 O 3 ) 1.5 , and its X-ray diffraction pattern is shown in Figure 2. The weight percentage of the catalyst is: 15.0% of cobalt oxide and 85.0% of alu...
example 2
[0030] Weigh 2.343g Co(NO 3 ) 2 ·6H 2 O, 4.202g Fe(NO 3 ) 3 9H2O and 18.881g Al(NO 3 ) 3 ·9H 2 O, add 68.7 ml of deionized water to make solution #1. Weigh out 22.012g NaOH and 3.645g Na 2 CO 3 , 584.7 ml of deionized water was added to prepare precipitant solution #2. The subsequent steps are the same as in Reference Example 1, and the typical structure of the hydrotalcite-like precursor is shown in Figure 1; after calcination, a CDUT-CAF-20 catalyst is obtained, which is composed of (Fe 2 O 3 ) 0.21 (Co 3 O 4 ) 0.16 (Al 2 O 3 ) 1.5 , and its typical structure is shown in Figure 2. The weight percentage of the catalyst is: 15.1% of cobalt oxide, 20.8% of iron oxide, and 64.1% of aluminum oxide.
[0031] The CDUT-CAF-20 catalyst was tested for the activity of acetic acid autothermal reforming, and the reaction conditions were the same as those of Reference Example 1. The acetic acid conversion rate of the catalyst was 85.46%, and the hydrogen yield was low, ...
Embodiment 1
[0033] Weigh 2.364g Co(NO 3 ) 2 ·6H 2 O, 2.188g Fe(NO3)3 9H2O and 23.543g Al(NO3 3 ) 3 ·9H 2 O, add 71.5 ml of deionized water to make solution #1. Weigh out 22.904g NaOH and 3.793g Na 2 CO 3 , and 608.4 ml of deionized water was added to make solution #2. The subsequent steps are the same as in Reference Example 1, and the typical structure of the hydrotalcite-like precursor is shown in Figure 1; after calcination, a CDUT-CAF-10 catalyst is obtained, which is composed of (Fe 2 O 3 ) 0.14 (Co 3 O 4 ) 0.14 (Al 2 O 3 ) 1.5 , and its typical structure is shown in Figure 2. The weight percentage of the catalyst is: 15.2% of cobalt oxide, 10.8% of iron oxide, and 74% of alumina.
[0034] The CDUT-CAF-10 catalyst was tested for the activity of autothermal reforming of acetic acid, and the reaction conditions were the same as those of Reference Example 1. The acetic acid conversion of the catalyst is stable at 100%, and the hydrogen yield is stable at about 2.41mol-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com