Coenzyme Q10 and octacosanol compounded type nanostructure lipid carrier and preparation method thereof
A technology of nanostructured lipids and octacosanol, which can be used in pharmaceutical formulations, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of low drug loading rate and unpublished particle size of aqueous solution And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] oxidized coenzyme Q 10 2 parts, 1 part of octacosanol, 1 part of caprylyl capric acid glyceride (ODO), 0.001 part of vitamin E, 1 part of decaglyceryl laurate, 1 part of decaglyceryl stearate.
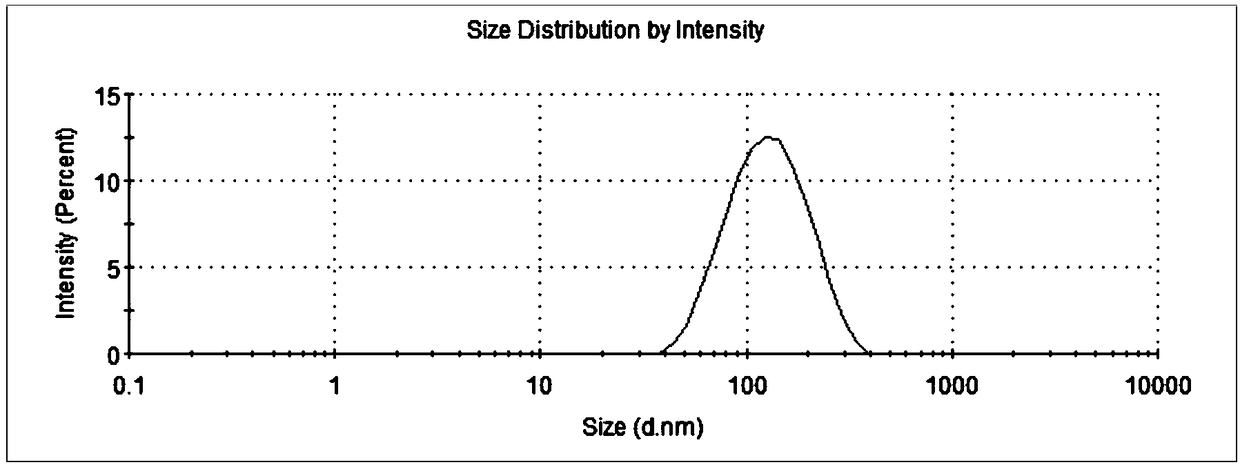
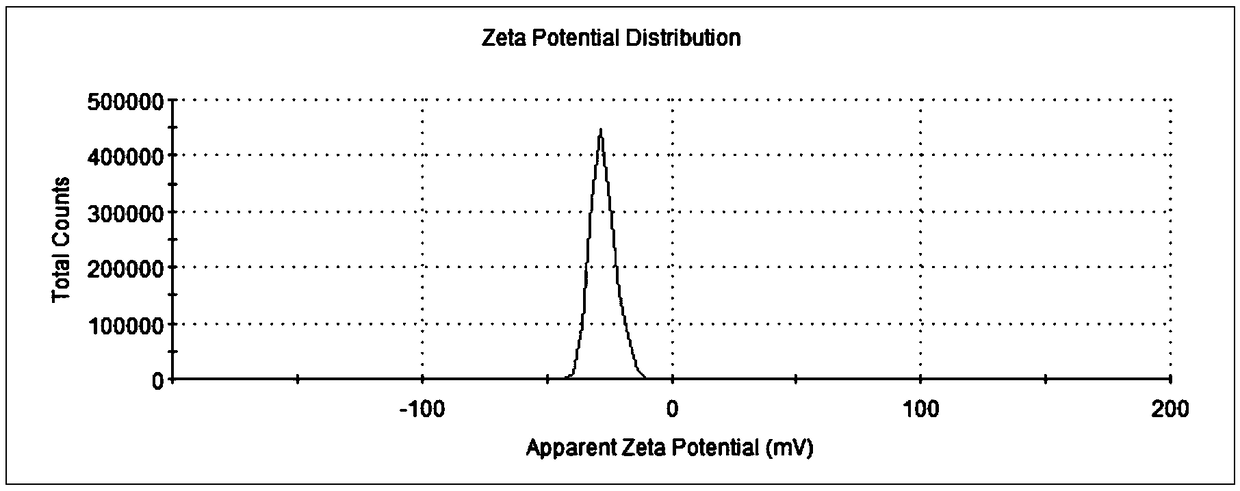
[0037] Weigh 4g oxidized coenzyme Q 10 . Add polyglycerol stearate to 200mL water at 90°C, stir and mix well to form a water phase; slowly add the above oil phase to the water phase, and use a shearer to cut at 16000rpm for 1 minute to form colostrum; then use high pressure Homogenizer, under the pressure of 60MP, through N 2 protection, after 5 cycles, an emulsion is formed, cooled to room temperature, and a nanostructured lipid carrier aqueous solution is obtained. After determination, the average particle size is 112.9nm, the potential is (-27.5±4.99)mV, coenzyme Q 10 The drug loading rate and encapsulation efficiency of the drug were 32.5% and 97.4%, respectively, and the drug loading rate and encapsulation efficiency of octacosanol were 15.9% and 95.3%, respectively.
Embodiment 2
[0039]oxidized coenzyme Q 10 1 part, 2 parts of octacosanol, 1 part of caprylic and capric glyceride (ODO), 0.002 parts of vitamin E, 1.5 parts of Tween-80, 1.5 parts of soybean lecithin.
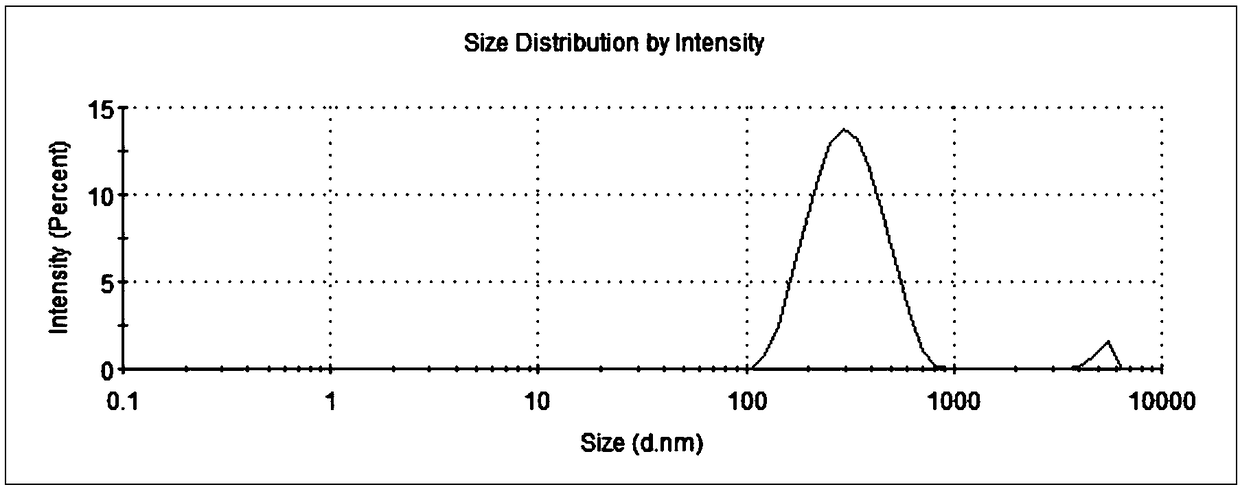
[0040] Weigh 2g oxidized coenzyme Q 10 , 4g octacosanol, 2g caprylic acid glyceride (ODO), 0.004g vitamin E were heated and melted at 85°C, stirred and mixed to form an oil phase; 3g Tween-80, 3g soybean lecithin were weighed and added Stir and mix in 250mL water at 85°C to form a water phase; slowly add the above oil phase to the water phase, and use a shearer to cut at 16000rpm for 1.5 minutes to form colostrum; then use a high-pressure homogenizer to Under pressure, pass N 2 Protection, after 7 cycles, an emulsion is formed, rapidly cooled to room temperature, and an aqueous solution of a nanostructured lipid carrier is obtained. After determination, the average particle size is 210.6nm, coenzyme Q 10 The drug loading rate and encapsulation efficiency of the drug were 13.6% and 95.5%...
Embodiment 3
[0042] oxidized coenzyme Q 10 1 part, 1 part of C22-C36 higher fatty alkanol mixture containing 70% octacosanol, 0.5 part of caprylic and capric glyceride (ODO), 0.003 part of vitamin E, 0.2 part of soybean lecithin, polyethylene glycol Alcohol-12-hydroxystearate (HS15) 1.8 parts.
[0043] Weigh 2g oxidized coenzyme Q 10 , 2g of C22-C36 higher fatty alkanol mixture containing 70% octacosanol, 1g of caprylic, capric acid glyceride (ODO), 0.006g of vitamin E were heated and melted at 70°C, stirred and mixed to form an oil phase; Weigh 0.4g soybean lecithin, 3.6g polyethylene glycol-12-hydroxystearate (HS15) into 150mL water at 70°C, stir and mix well to form a water phase; slowly add the above oil phase to the water phase, use Shearing machine, after shearing 2 minutes with 16000rpm, forms colostrum; Then use high-pressure homogenizer, under the pressure of 30MP, pass N 2 protection, after 10 cycles, an emulsion is formed, rapidly cooled to room temperature, and a nanostructu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com