Electrocatalyst for catalyzing water decomposition to produce hydrogen, and preparation method and application thereof
An electrocatalyst and catalyst technology, applied in catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc., can solve problems such as harsh operating conditions and complex processes, and achieve high reaction efficiency, simple operation, and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
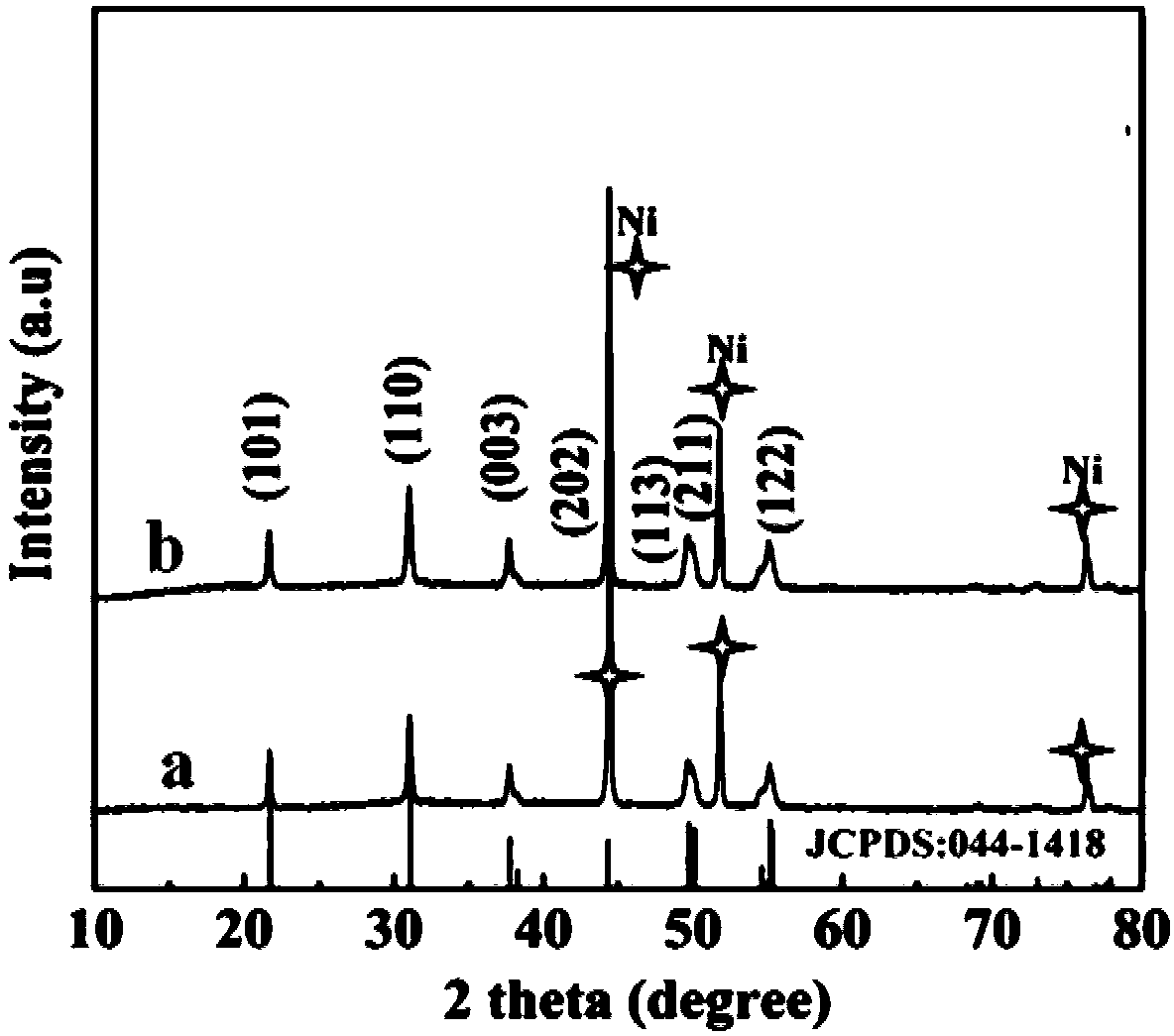
[0031] Ni 3 S 2 The specific steps of @NiOOH@NF catalyst preparation: First, put the treated nickel foam vertically into the catalyst containing 0.3M Na 2 In a reaction kettle of S aqueous solution, put it in an oven at 120°C for hydrothermal reaction for about 10 hours to form Ni 3 S 2 @NF Catalyst. References: N. Jiang, Q. Tang, M. Sheng, B. You, D. Jiang, Y. Sun, Nickel sulfides for electrocatalytic hydrogen evolution under alkaline conditions: a case study of crystalline NiS, NiS 2 , and Ni 3 S 2 nanoparticles, Catal. Sci. Technol. 2016, 6, 1077–1084. Rinse the sulfided nickel foam several times with deionized water and dry it naturally, carry out electrochemical oxidation in a borate buffer solution, apply a bias voltage of 1.2V, and use the Ni obtained above 3 S 2 @NF is used as working electrode, Ag / AgCl is used as reference electrode, platinum mesh is used as counter electrode, and borate buffer solution is used as electrolyte to obtain Ni after electrodepositi...
Embodiment 2
[0034] Perform electrochemical oxidation on the sulfided nickel foam, apply a bias voltage of 1.2V, and use Ni 3 S 2 @NF is the working electrode, Ag / AgCl is the reference electrode, the platinum mesh is the counter electrode, and boric acid buffer solution (pH 9.18) is the electrolyte, respectively depositing 0, 200, 400, 600, 800, 1000s, through linear sweep voltammetry (LSV) test to illustrate the effect of deposition time on catalyst activity (see Figure 5 ).
[0035] Depend on Figure 5 Displayed Ni 3 S 2 LSV curves of electrodeposited NiOOH at different deposition times for @NF electrodes. It can be observed that the HER activity of the synthesized electrodes is affected by the deposition time. Among them, the activity was the best when the electrodeposition was 800s, and no further increase in activity was observed beyond this time. Therefore, the synthesis of Ni 3 S 2 The deposition time of @NiOOH@NF catalyst was optimized to be 800s.
Embodiment 3
[0037] Made Ni 3 S 2 Catalytic activity evaluation of @NiOOH@NF catalyst.
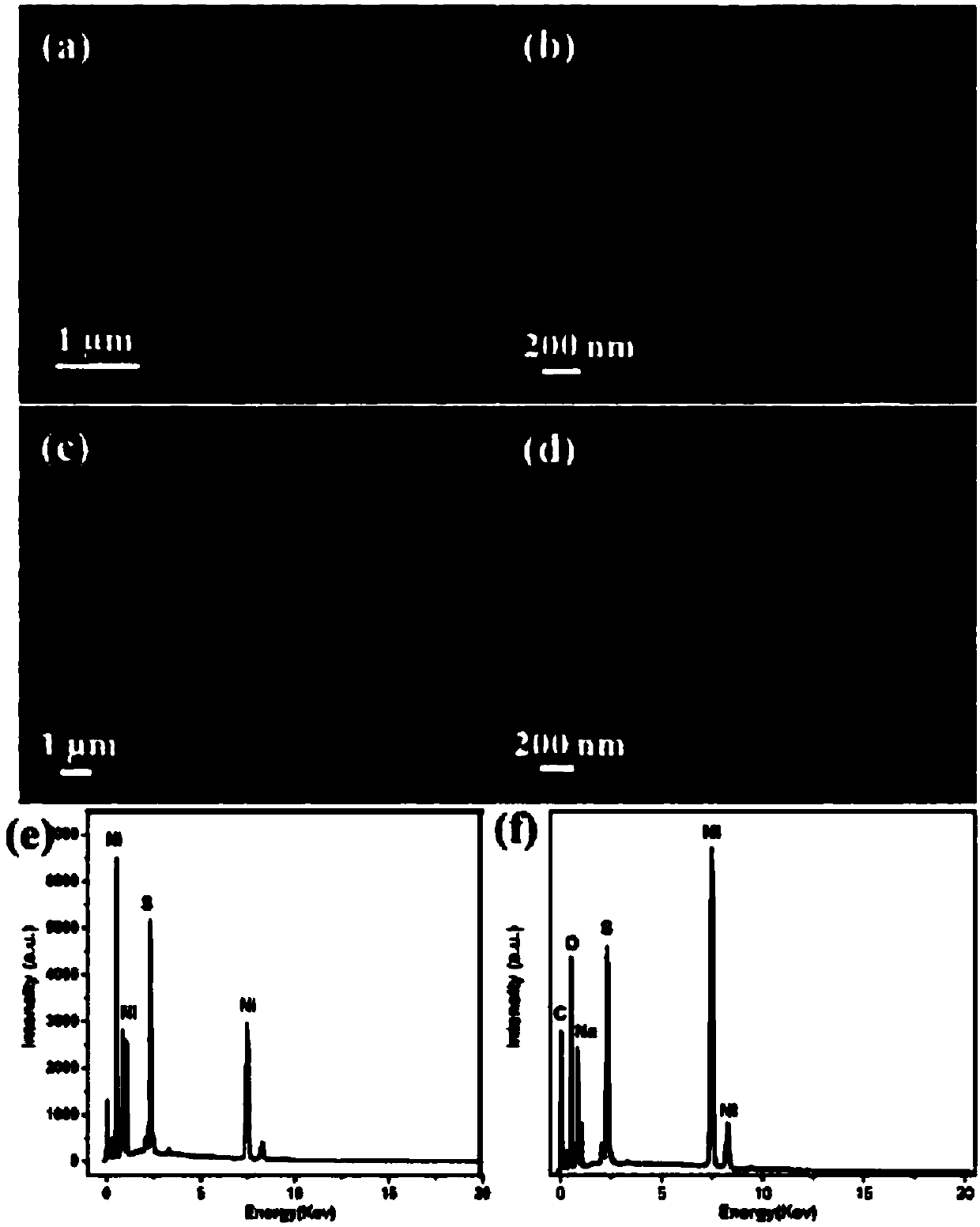
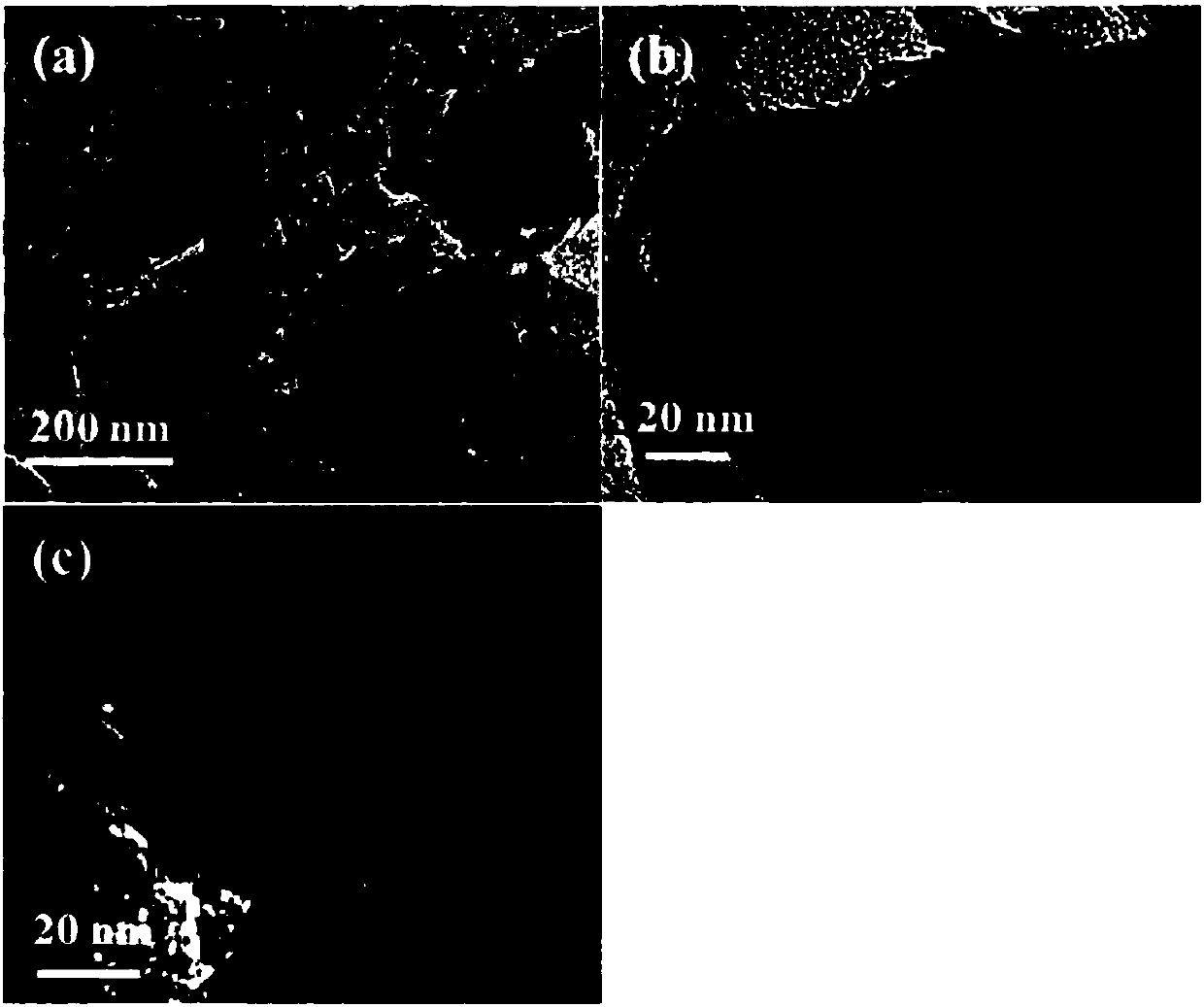
[0038] With 1M NaOH as the electrolyte solution, Ag / AgCl as the reference electrode, and platinum mesh as the counter electrode, NF, NiOOH@NF, Ni 3 S 2 @NF,Ni 3 S 2 @NiOOH@NF is the working electrode, the scan rate is 5mV / s, and the LSV test is carried out. The catalytic activities of different electrodes are as follows Image 6 shown.
[0039] Such as Image 6 As shown in (a), compare Ni in 1M NaOH solution 3 S 2 @NF and Ni 3 S 2 HER performance of @NiOOH@NF catalysts. Meanwhile, the HER performance of bare NF and NiOOH@NF was also tested using the same method. Ni 3 S 2 @NiOOH@NF at 10mA / cm 2 The lowest overpotential is 160mV when the Ni 3 S 2 @NiOOH@NF has the best catalytic activity for HER. In contrast, NF, NiOOH@NF and Ni 3 S 2 @NF sample at 10mA / cm 2 The overpotentials of 217, 210 and 237mV were displayed respectively. By comparing bare NF, NiOOH@NF, Ni 3 S 2 @NF and Ni 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com