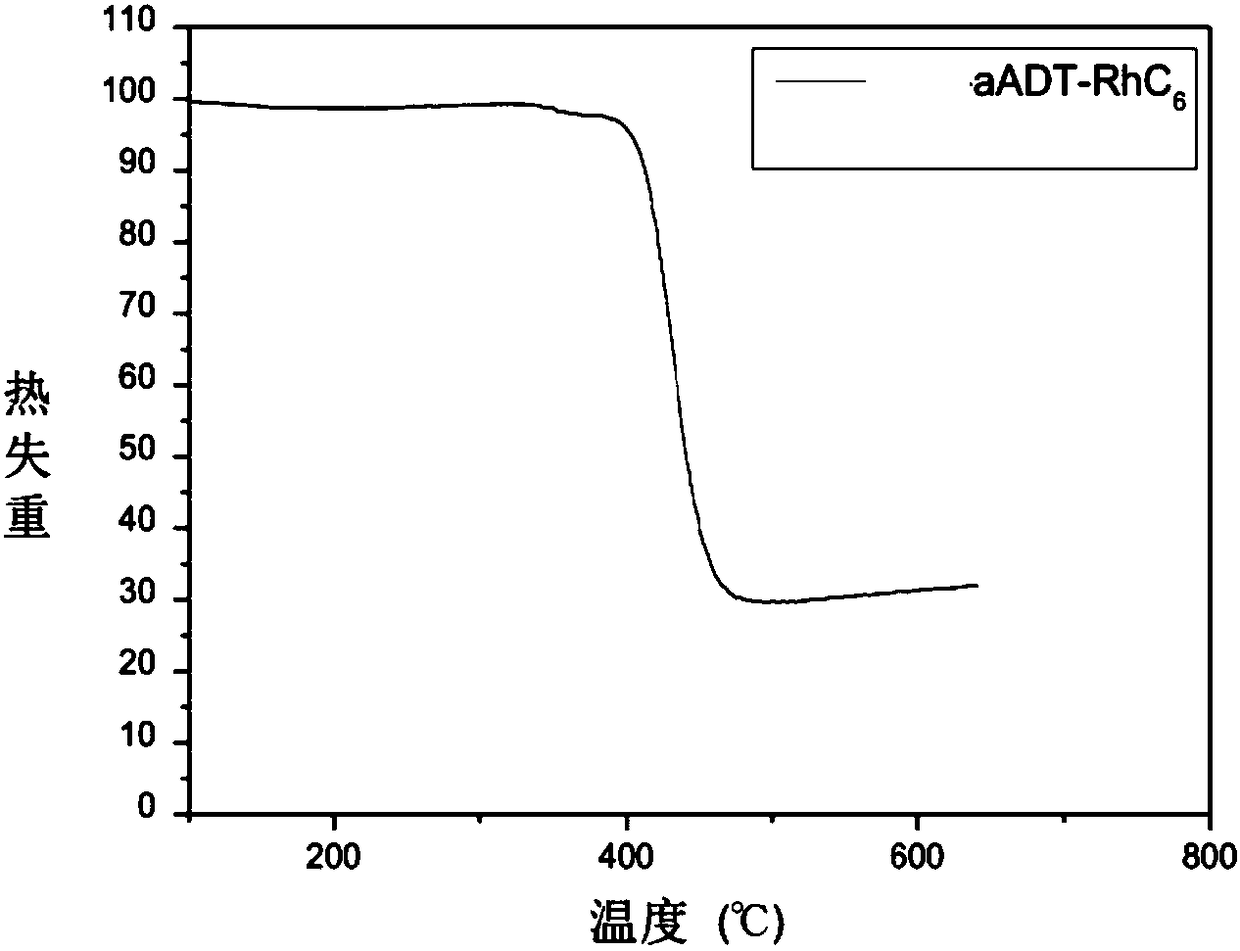
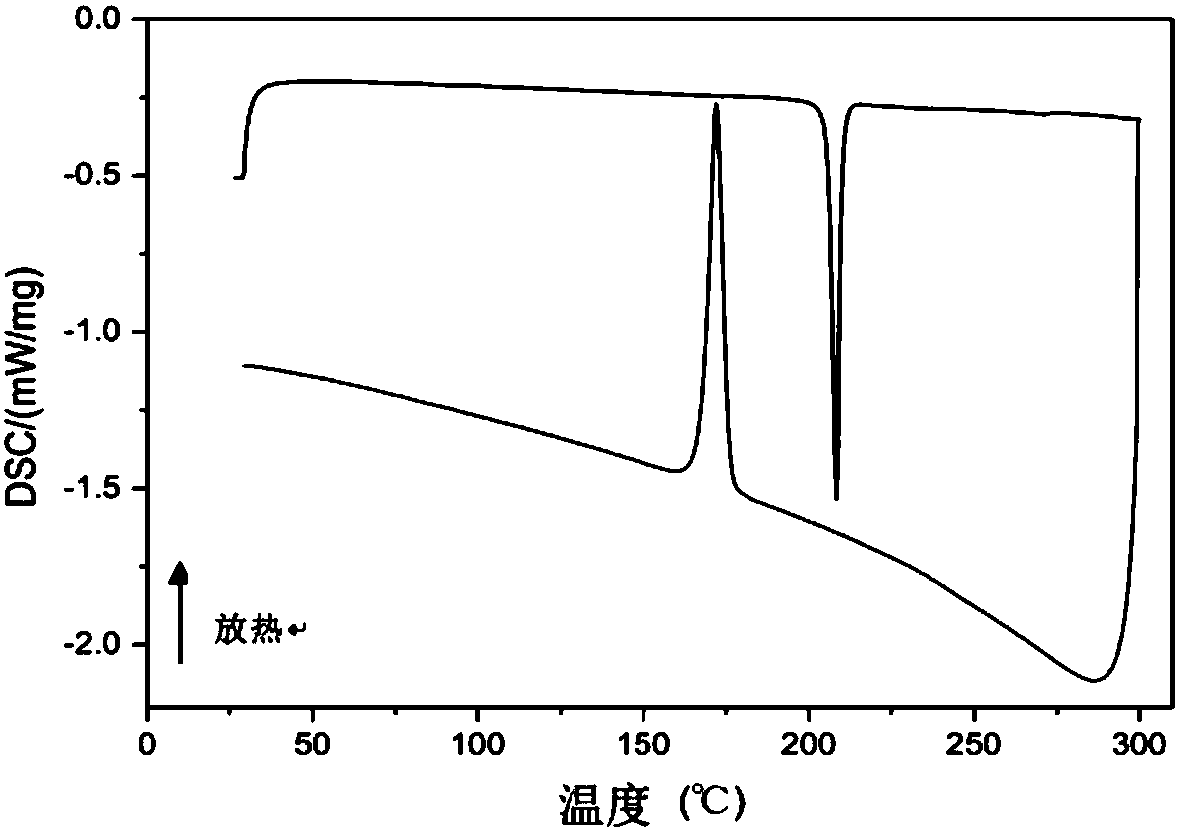
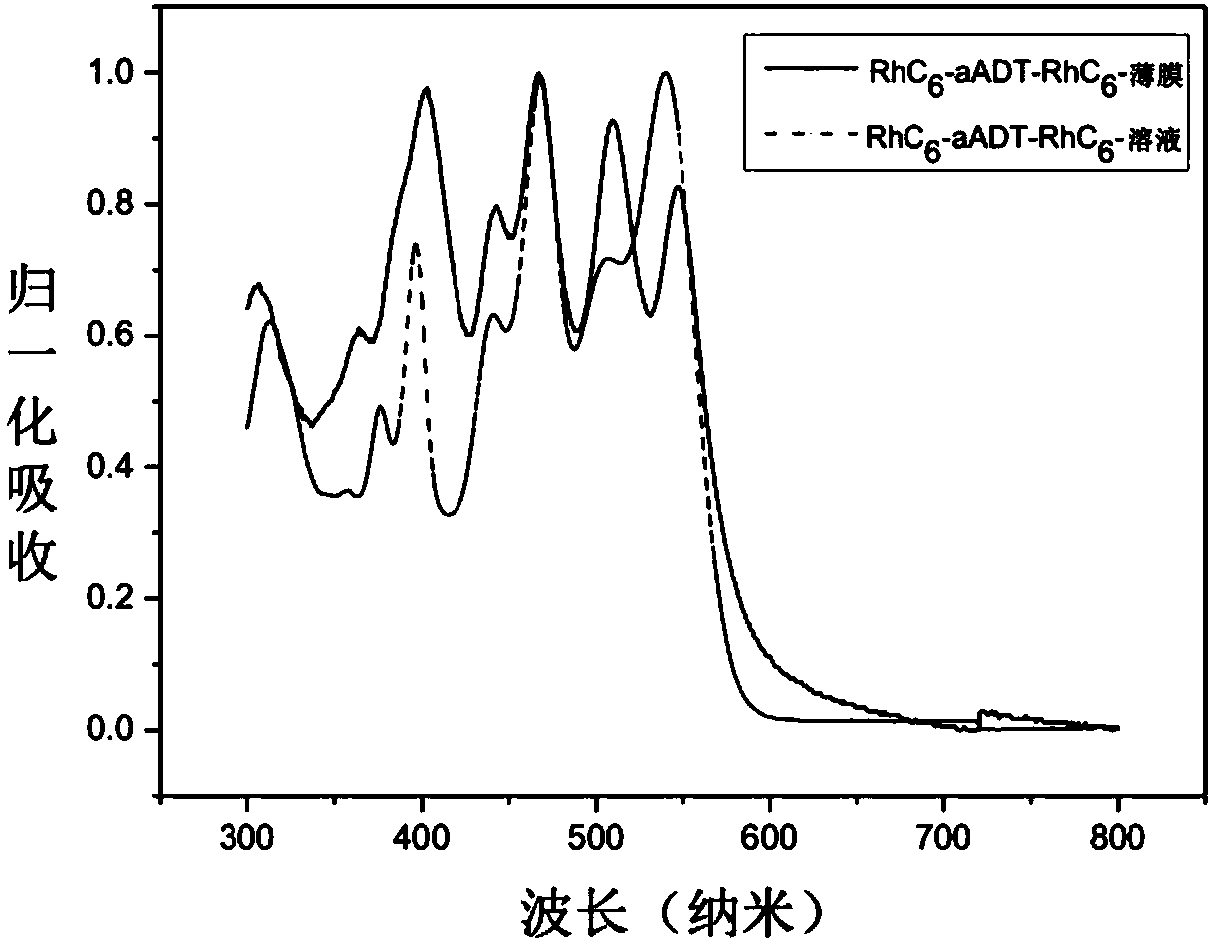
Organic small molecular semiconductor material containing anthracene dithiophene as well as preparation method and application thereof
A bithiophene and semiconductor technology, applied in the field of organic optoelectronics, can solve the problems of high cost of raw materials, easy aggregation, difficulty in preparation and purification, etc., and achieve the effects of good device performance, high electron mobility, and good light harvesting ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、1
[0028] Embodiment 1, the synthesis of 1,4-dibromo-2,5-diiodobenzene
[0029]
[0030] Add 1,4-dibromobenzene (10.00g, 42.39mmol) and simple iodine (43.04g, 169.56mmol) into a 500ml one-necked flask, and slowly add 200ml of concentrated sulfuric acid at room temperature. Install a spherical condenser on the top of the single-necked flask, heat the flask in an oil bath at 125°C, turn on the condensed water, make it reflux, and react like this for 3 days. The color of the solution gradually changed from initial purple to dark purple to black. The final product was white mixed with black, and the solution was black. Take a 1000ml large beaker, add a large amount of ice cubes, slowly pour the reaction solution into it, stir to cool it down, filter it with a suction filter, and use NaHCO 3 and Na 2 S 2 o 3 The solution was washed repeatedly to remove I 2 , the suction filtration device sucked the filter residue, heated and dissolved the filter residue with DCM, filtered and...
Embodiment 2
[0032] Embodiment 2, the synthesis of 2-(4-bromothien-2-yl)-1,3-dioxolane
[0033]
[0034] Add 4-bromothiophene-2-carbaldehyde (5g, 26.17mmol), ethylene glycol (3.25g, 52.35mmol), 200mg of p-toluenesulfonic acid as a catalyst, and 100ml of toluene as a solvent in a single-necked flask. The water tank removes the water generated by the reaction. Through condensed water, heated to reflux for 8h. After the reaction was completed, p-toluenesulfonic acid was removed with NaOH solution, extracted, and the upper layer was the desired product. Then pass through the column, the collected sample solution is light yellow, 6.10 g, and the yield is 99.2%.
[0035] 1 H NMR (500MHz, CDCl3) δ7.20(s,1H),7.00(s,1H),5.58(s,1H),3.35(s,4H).
Embodiment 3
[0036] Embodiment 3, the synthesis of (3-bromo-5-(1,3-dioxolan-2-yl) thiophen-2-yl) tributylstannane
[0037]
[0038] Add 2-(4-bromothiophen-2-yl)-1,3-dioxolane (3.68g, 15.65mmol) into a two-necked flask, pump and ventilate 3 times, use a 50ml syringe to extract 20ml of anhydrous THF, Pour into a two-necked flask. Allow it to stir at -20°C for 10 minutes, ventilate, take 17.22ml (17.22mmol) LDA with a long needle, and add it dropwise in a two-necked flask. The color of the solution changes from light yellow to reddish brown, then dark red, and finally turns black. Allow it to react at -20°C for 2 hours, then take 4.56ml (1.118g / ml) tributyltin chloride into the reaction solution with a long needle, return to normal temperature, and react overnight to obtain a light yellow transparent viscous liquid. Extracted 3 times with n-hexane, washed 3 times with NaCl aqueous solution, filtered and rotary evaporated to remove the solvent, and then dried the solvent with a diaphragm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical bandgap | aaaaa | aaaaa |
| Short circuit current density | aaaaa | aaaaa |
| Open circuit voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com