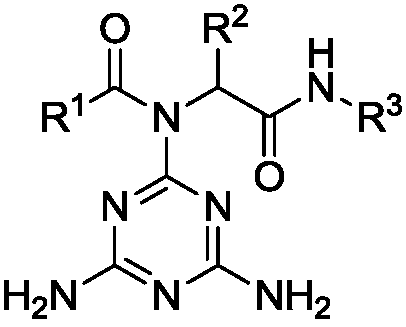
Flame retardants containing melamine structure as well as preparation method and application thereof
A melamine and flame retardant technology, applied in the field of flame retardants, can solve the problems of slow application and development of flame retardants, complex synthesis process, low yield, etc., and achieve high use value, high reaction efficiency and low preparation cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0032] Hereinafter, the present invention will be further described in conjunction with the embodiments, but the scope of protection claimed by the present invention is not limited to the scope expressed by the embodiments.
[0033] Instruments and reagents:
[0034] The melting point was measured with X4 melting point meter (produced by Beijing No. 3 Optical Instrument Factory), and the thermometer was not calibrated; 1 H NMR and 13 C NMR is measured by Varian Mercury 400 400MHz nuclear magnetic resonance instrument or Varian Mercury 600 600MHz nuclear magnetic resonance instrument, deuterated chloroform (CDCl 3 ) Or deuterated dimethyl sulfoxide (DMSO-d 6 ) Is the solvent and TMS is the internal standard; MS is determined by FinniganTrace mass spectrometer; elemental analysis is determined by Vario EL III elemental analyzer; the reagents used are domestic (or imported) chemical or analytical pure. The solvents methanol and isopropanol are dried.
Embodiment 1
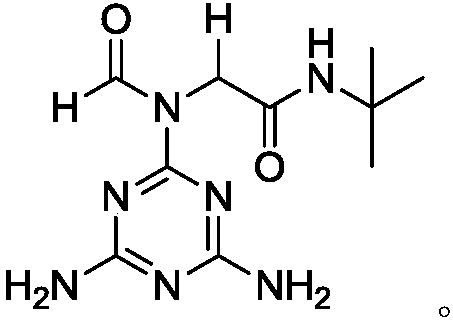
[0036] A method for preparing N-(tert-butyl)-2-(N-(4,6-diamino-1,3,5-triazin-2-yl)formamido)acetamide flame retardant includes the following experimental steps:
[0037] Under the protection of nitrogen, the compound melamine 1 (0.378g, 3mmol, 3.0eqv.), formic acid 2a (0.046g, 1.0mmol, 1.0eqv.), and formaldehyde 3a (0.030g, 1.0mmol, 1.0eqv.) were added to the reactor in sequence. ), tert-butyl isonitrile 4a (0.091g, 1.1mmol, 1.1eqv.), MeOH:i-PrOH=2:1 (10ml), heated to 50°C and stirred for 30 minutes to dissolve. After dissolving, add the copper complex supported by the catalyst MCM-41 (0.025g), MIL-101 (0.025g) and p-toluenesulfonic acid (0.017g) in sequence. Continue stirring, while the temperature drops to 40℃. After the reaction continues for 24 hours, TLC After the detection reaction is completed, the solvents methanol and ethanol are removed under reduced pressure, and the remainder is filtered, extracted and column chromatography to obtain the target flame retardant Ia with...
Embodiment 2
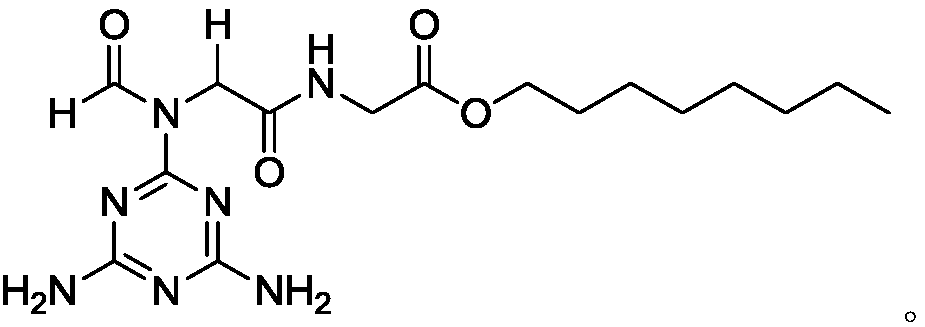
[0045] A method for preparing octyl 2-(2-(N-(4,6-diamino-1,3,5-triazin-2-yl)formamido)acetamido)acetate flame retardant includes the following experimental steps:
[0046] Under the protection of nitrogen, the compound melamine 1 (0.378g, 3mmol, 3.0eqv.), formic acid 2a (0.046g, 1.0mmol, 1.0eqv.), and formaldehyde 3a (0.030g, 1.0mmol, 1.0eqv.) were added to the reactor in sequence. ), n-octyl acetate isonitrile 4b (0.22 g, 1.1 mmol, 1.1 eqv.), MeOH: i-PrOH=2:1 (10 ml), heated to 50° C. and stirred for 30 minutes to dissolve. After dissolving, add the copper complex supported by the catalyst MCM-41 (0.025g), MIL-101 (0.025g) and p-toluenesulfonic acid (0.017g) in sequence. Continue stirring, while the temperature drops to 40℃. After the reaction continues for 24 hours, TLC After the detection reaction is completed, the solvents methanol and ethanol are removed under reduced pressure, and the remainder is filtered, extracted and column chromatography to obtain the target flame reta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com