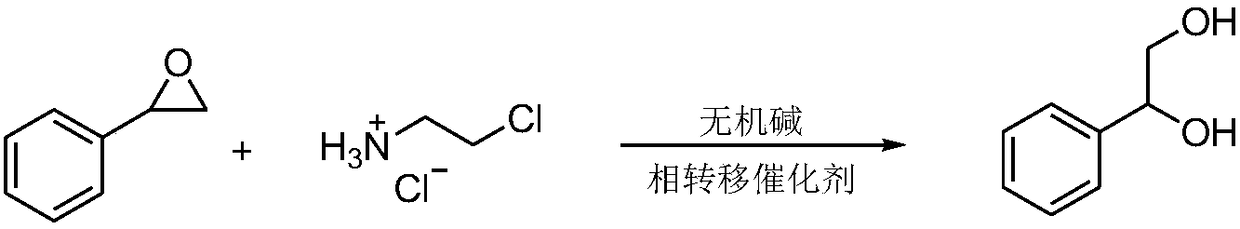
Synthetic method for 1-phenyl-1,2-ethanediol
A technology of phenylethylene glycol and its synthesis method, which is applied in the fields of chemical instruments and methods, preparation of hydroxyl compounds, and preparation of organic compounds, etc., and can solve the problems of not meeting the requirements of green chemical development, low catalytic reaction efficiency, and difficulty in obtaining catalysts and other problems, to achieve the effect of easy to obtain catalyst, reduce industrial three wastes, and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Preparation of phenyl glycol
[0019] (1) Reaction: Add 5 g (41.67 mmol) of styrene oxide, 5 mg of tetrabutylammonium bromide, 0.48 g (4.17 mmol) of 2-chloroethylamine hydrochloride, and carbonic acid in a flask with a thermometer and reflux condenser. 0.34g (4.17mmol) of sodium hydride, 50mL of water, stirred, heated to reflux, and the reaction temperature was 40°C;
[0020] (2) Endpoint monitoring: The reaction in step (1) is monitored by HPLC, and the end point of the reaction is when the raw material styrene oxide disappears;
[0021] (3) Extraction: When the reaction in step (1) reaches the end of the reaction, stop heating, cool the system to room temperature, and extract with 80 mL of ethyl acetate. Separate the ethyl acetate layer and desolvate under reduced pressure to obtain a white solid.
[0022] The yield of the obtained phenyl glycol is 99%, the purity is 98.5% (determined by HPLC), the melting point: 63-65°C, MS m / z: 161.05 (M+Na, 100).
Embodiment 2
[0023] Example 2: Preparation of Phenyl Glycol
[0024] (1) Reaction: Add 10g (83.34mmol) of styrene oxide, 12mg of tetrabutylammonium bromide, 0.7g (6.03mmol) of 2-chloroethylamine hydrochloride, and carbonic acid in a flask with a thermometer and reflux condenser. 0.506g (6.03mmol) of sodium hydride, 120mL of water, stirred, heated to reflux, and the reaction temperature was 45°C;
[0025] (2) Endpoint monitoring: The reaction in step (1) is monitored by HPLC, and the end point of the reaction is when the raw material styrene oxide disappears;
[0026] (3) Extraction: When the reaction in step (1) reaches the end of the reaction, stop heating, cool the system to room temperature, and extract with 120 mL of ethyl acetate. Separate the ethyl acetate layer and desolvate under reduced pressure to obtain a white solid.
[0027] The yield of the obtained phenyl glycol is 97%, the purity is 96% (determined by HPLC), the melting point: 63-65° C., MS m / z: 161.05 (M+Na, 100).
Embodiment 3
[0028] Example 3: Preparation of Phenyl Glycol
[0029] (1) Reaction: Add 3g (25mmol) of styrene oxide, 24mg of tetrabutylammonium bromide, 0.29g (2.5mmol) of 2-chloroethylamine hydrochloride, and hydrogen carbonate in a flask with a thermometer and reflux condenser. 0.21g (2.5mmol) of sodium, 60mL of water, stirred, heated to reflux, and the reaction temperature was 37°C;
[0030] (2) Endpoint monitoring: The reaction of step (1) is monitored by HPLC, and the end point of the reaction is when the raw material styrene oxide disappears;
[0031] (3) Extraction: When the reaction in step (1) reaches the end of the reaction, stop heating, cool the system to room temperature, extract with 60 mL of ethyl acetate, separate the ethyl acetate layer, and desolvate under reduced pressure to obtain a white solid.
[0032] The yield of the prepared phenyl glycol is 96%, the purity is 98% (determined by HPLC), the melting point: 63-65° C., MS m / z: 161.05 (M+Na, 100).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com