Preparation method and application of anticoagulant and anti-adhesion polyheptafluorobutyl acrylate-polycaprolactone block polymer nanofiber membrane
A technology of polyheptafluorobutyl acrylate and heptafluorobutyl acrylate, which is applied in anticoagulation treatment, fiber treatment, pharmaceutical formulations, etc., and can solve the problem of affecting the anticoagulation effect and biocompatibility of biological materials, modification or modified group uneven distribution, high production cost, etc., to achieve the effect of improving blood compatibility, controllable degradation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
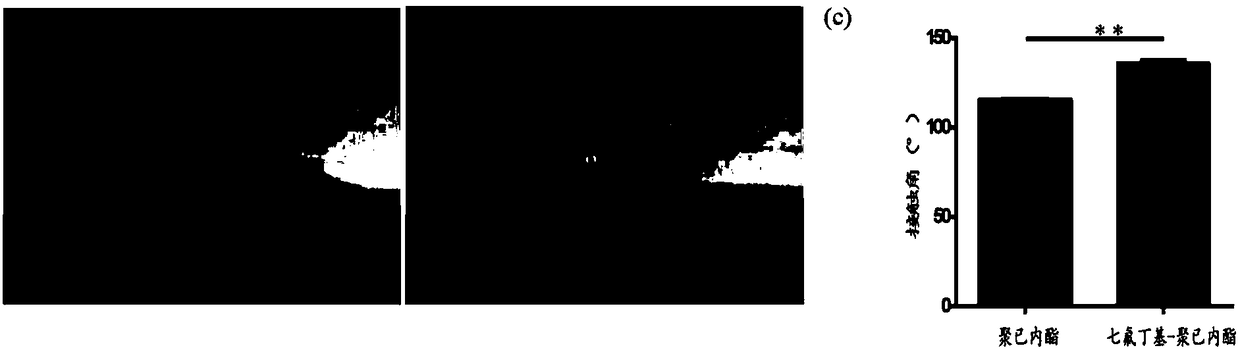
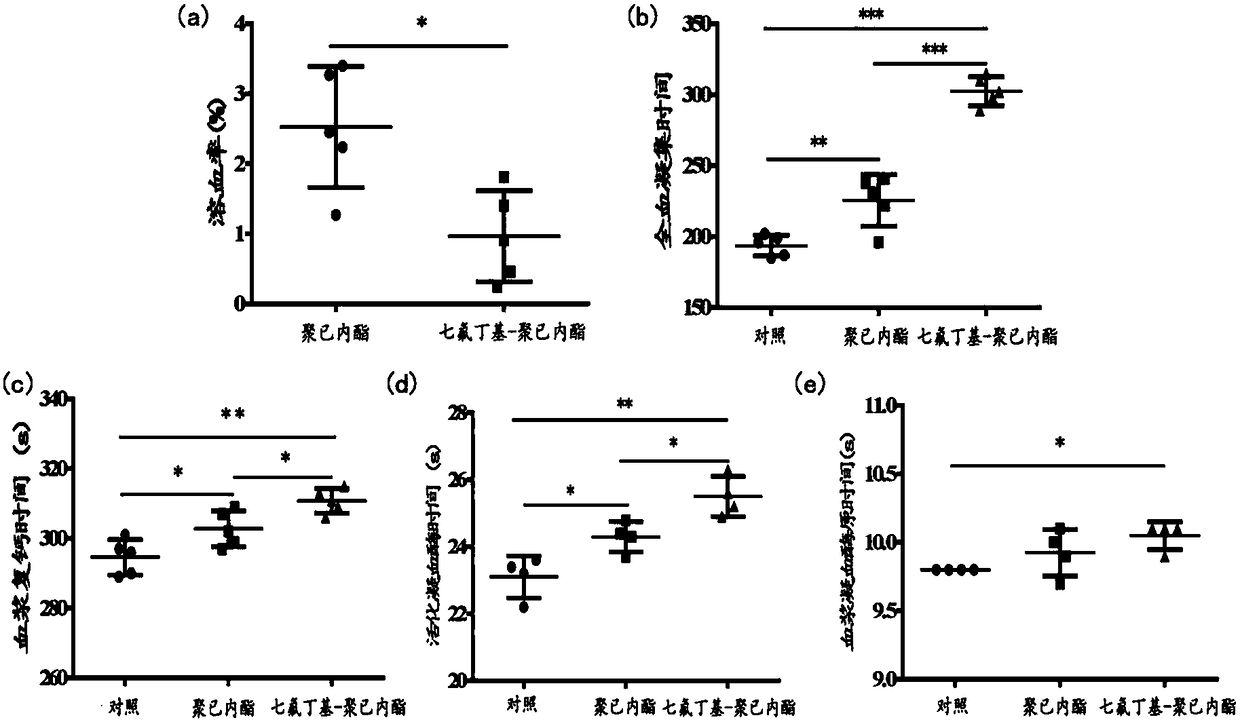
Image
Examples
Embodiment 1
[0034] Embodiment 1: A kind of preparation method of anticoagulant anti-adhesion polyheptafluorobutyl acrylate-polycaprolactone block polymer nanofiber membrane comprises the following steps:
[0035](1) Synthesis of trithiocarbonate-terminated polycaprolactone: 5g polycaprolactone and 0.182g S-dodecyl-S'-(α,α'-dimethyl-α"-acetic acid ) Trithiocarbonate was put into a 100mL flask, and heated under vacuum at 80°C for 2 hours to remove moisture. Then add 30mg dimethylaminopyridine and 95mg 1-(3-dimethylaminopropyl)-3-ethylcarbodi imine hydrochloride) was degassed by bubbling in nitrogen for 20min, and 60mL of anhydrous dichloromethane was added under nitrogen protection. After the polycaprolactone was dissolved, the solution was stirred at 40°C for 2 days, and the product was precipitated with 300mL of methanol , the product was redissolved in dichloromethane and precipitated again, repeated twice, and the resulting final product was collected by vacuum drying;
[0036] (2) Syn...
Embodiment 2
[0040] Embodiment 2: A method for preparing an anticoagulant and anti-adhesion polyheptafluorobutyl acrylate-polycaprolactone block polymer nanofiber membrane, comprising the following steps:
[0041] (1) Synthesis of trithiocarbonate-terminated polycaprolactone: 2 g of polycaprolactone and 72.8 mg of S-dodecyl-S'-(α,α'-dimethyl-α"-acetic acid ) Trithiocarbonate was put into a 50mL flask, and heated under vacuum at 70°C for 4 hours to remove moisture. Then add 12mg dimethylaminopyridine and 32mg 1-(3-dimethylaminopropyl)-3-ethylcarbodi imine hydrochloride) was degassed by bubbling in nitrogen for 20 minutes, and 20 mL of anhydrous dichloromethane was added under nitrogen protection. After the polycaprolactone was dissolved, the solution was stirred at room temperature for 2 days, and the product was precipitated with 100 mL of ethanol. The product was redissolved in dichloromethane and precipitated again, repeated twice, and the final product was collected under vacuum drying;...
Embodiment 3
[0045] Embodiment three: a kind of preparation method of anticoagulant antiadhesion polyheptafluorobutyl acrylate-polycaprolactone block polymer nanofiber membrane, comprises the following steps:
[0046] (1) Synthesis of trithiocarbonate-terminated polycaprolactone: 5g polycaprolactone and 0.182g S-dodecyl-S'-(α,α'-dimethyl-α"-acetic acid ) trithiocarbonate into a 100mL flask, heated at 60°C under vacuum for 6 hours to remove moisture. Then add dimethylaminopyridine and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride) was degassed by bubbling in nitrogen for 20min, and 60mL of anhydrous dichloromethane was added under nitrogen protection. After the polycaprolactone was dissolved, the solution was stirred at 30°C for 2 days, and the product was precipitated with isopropanol. The product was redissolved in dichloromethane and precipitated again, repeated twice, and the final product was collected under vacuum drying;
[0047] (2) Synthesis of hydrophobic polyheptaf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com