Construction method of HPLC characteristic chromatogram of Jingyaokang capsules
A construction method and a technology of characteristic maps, applied in the field of construction of HPLC characteristic maps, can solve problems such as controlling product quality, no relevant reports on construction methods, and difficulty in reflecting products as a whole, so as to ensure controllability, avoid singleness and One-sidedness, the effect of reducing the possibility of product quality compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
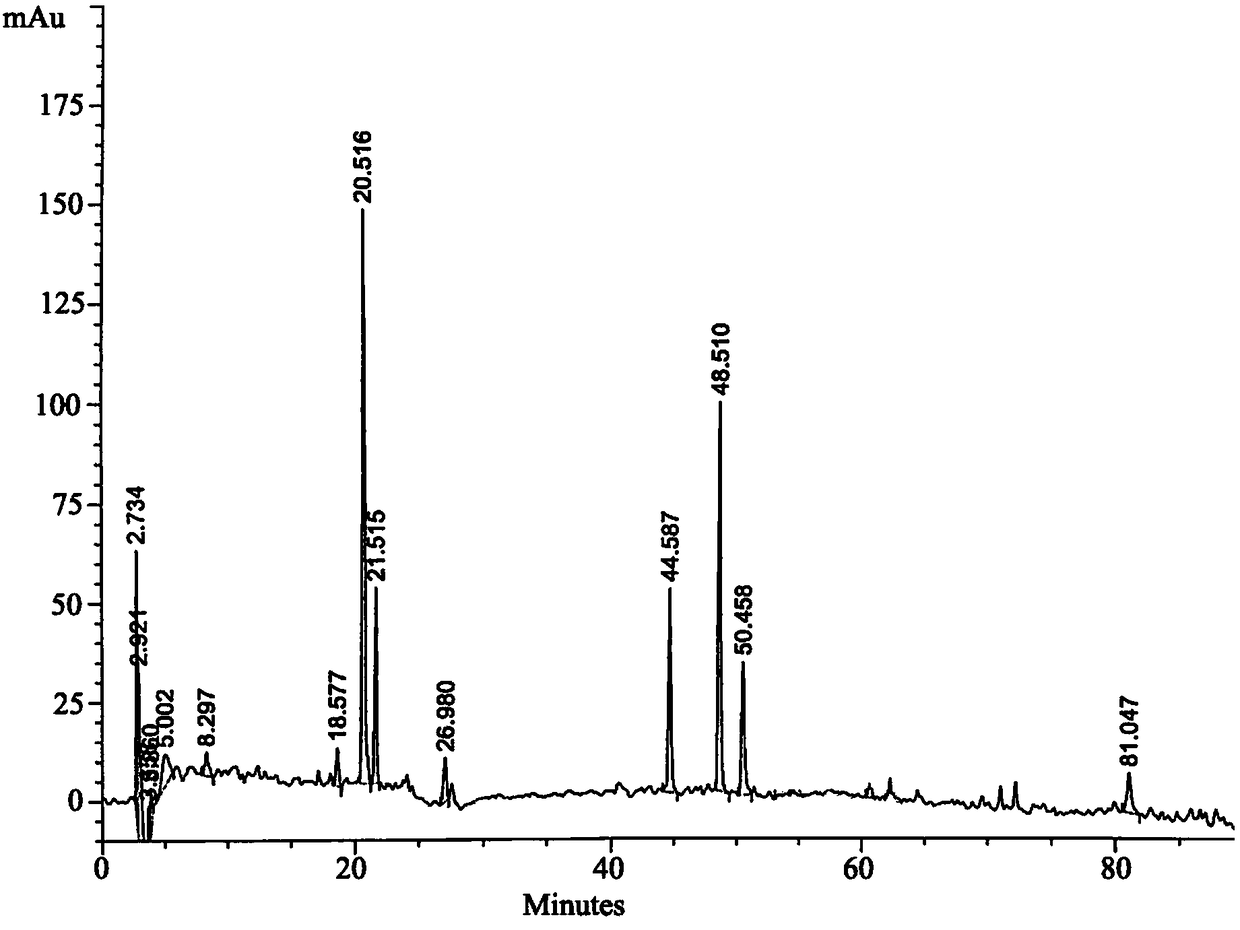
[0059] (1) Preparation of the test solution: take 2-5g of the content of this product, accurately weigh it, put it in a stoppered Erlenmeyer flask, add 20-50mL of 2%-10% methanolic hydrochloric acid solution precisely, weigh it, and heat Reflux extraction for 1 to 2 hours, or ultrasonic treatment for 20 to 40 minutes, let cool, weigh again, make up the lost weight with methanolic hydrochloric acid solution, shake well, filter, accurately measure 5 to 10 mL of the filtrate, and evaporate to dryness Add an appropriate amount of methanol to the residue to dissolve, add 2g of neutral alumina, mix well, evaporate to dryness, add to a neutral alumina column, 100-200 mesh, 6g, inner diameter 1.2cm, wash with 50mL of chloroform-methanol mixed solution The volume ratio of chloroform in the mixed solution is 50% to 90%. Collect the eluate, evaporate to dryness, add an appropriate amount of methanol to the residue to dissolve it, transfer it to a 10mL measuring bottle, add methanol to the...
Embodiment 1
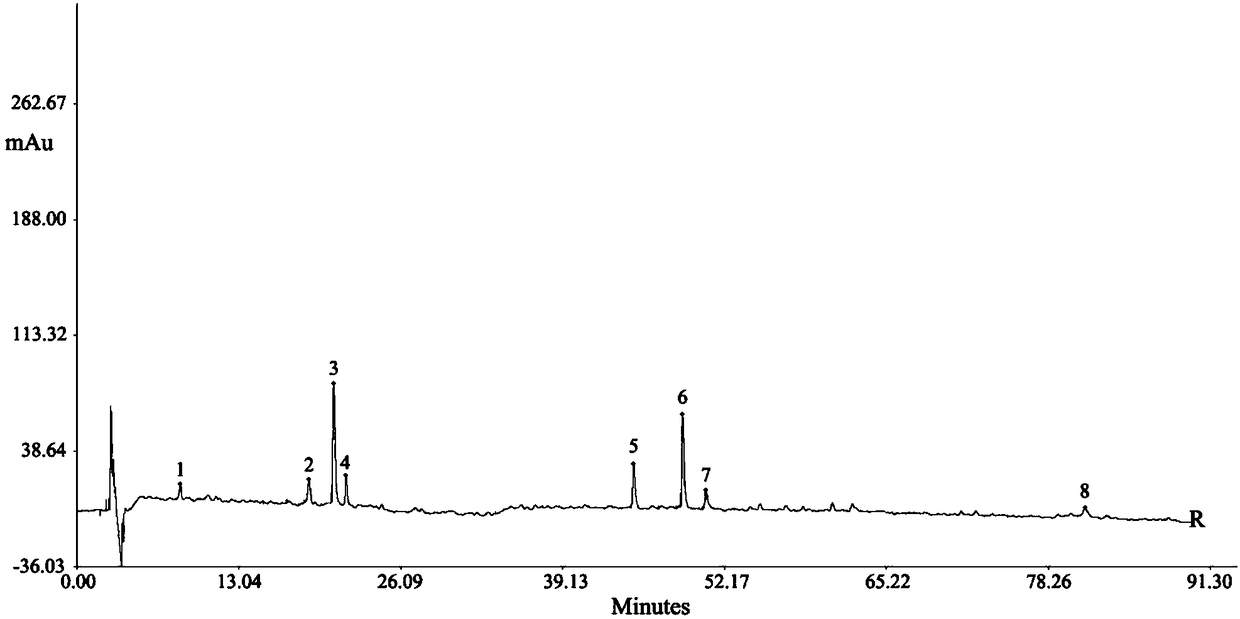
[0072] Example 1: Quality control method for Jingyaokang capsules based on characteristic maps
[0073] Instrument: Agilent1200 high performance liquid chromatograph, MS205DU analytical balance.
[0074] Reagents and reagents: strychnine reference substance (110705-201307), acetonitrile for liquid chromatography analysis is chromatographically pure, other reagents are analytically pure, water is ultrapure water, and Jingyaokang capsules are purchased from Jilin Changbaishan Pharmaceutical Group Co., Ltd. supply.
[0075] Preparation of the test solution: take about 5g of the content of this product, accurately weigh it, put it in a stoppered Erlenmeyer flask, add 50mL of 2% hydrochloric acid methanol accurately, weigh it, extract it under reflux for 1 hour, let it cool, and weigh it again Use hydrochloric acid methanol to make up for the lost weight, shake well, filter, accurately measure 10mL of the subsequent filtrate, evaporate to dryness, add appropriate amount of methano...
Embodiment 2
[0087] Example 2: Quality control method for Jingyaokang capsules based on characteristic maps
[0088] Instrument: Agilent1200 high performance liquid chromatograph, MS205DU analytical balance.
[0089] Reagents and reagents: strychnine reference substance (110705-201307), acetonitrile for liquid chromatography analysis is chromatographically pure, other reagents are analytically pure, water is ultrapure water, and Jingyaokang capsules are purchased from Jilin Changbaishan Pharmaceutical Group Co., Ltd. supply.
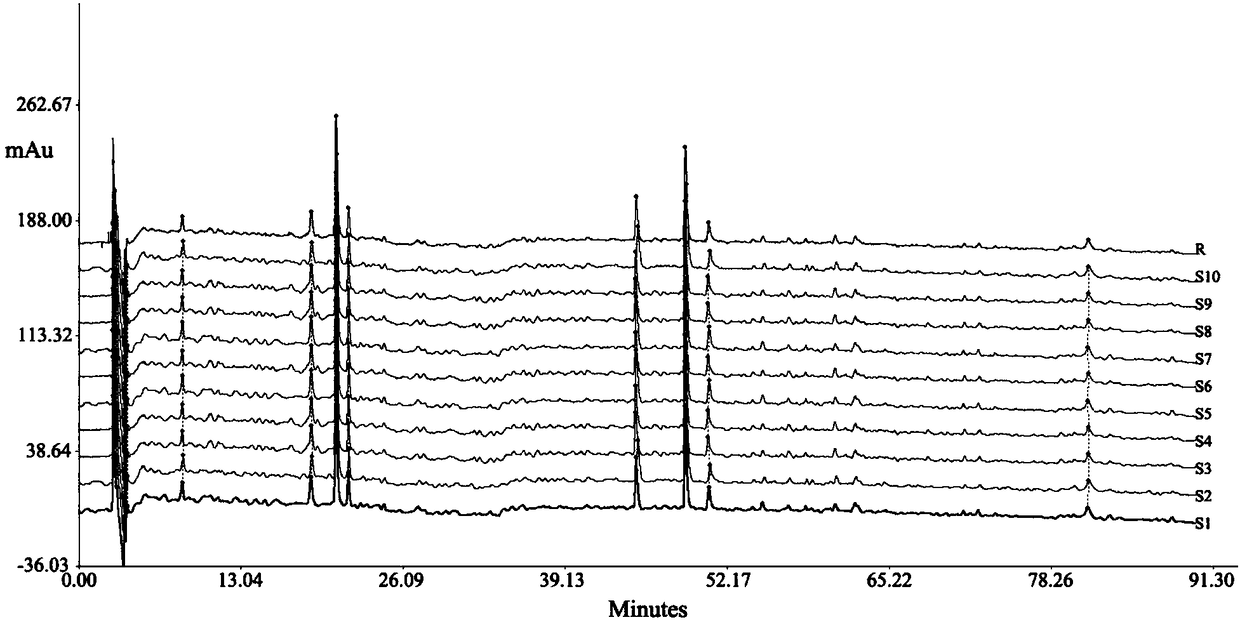
[0090] Preparation of the test solution: take about 5 g of the product, 6 parts, accurately weighed, respectively put in a stoppered Erlenmeyer flask, accurately add 50 mL of 2% hydrochloric acid methanol, weigh the weight, reflux extraction for 1 hour, let cool , weigh again, make up the lost weight with methanol hydrochloride, shake well, filter, accurately measure 10mL of the subsequent filtrate, evaporate to dryness, add appropriate amount of methanol to the res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Column length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com