Synthesis method of lenalidomide metabolite
A synthesis method and technology of lenalidomide are applied in the field of synthesis of lenalidomide metabolites to achieve the effects of reasonable process design, high purity and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
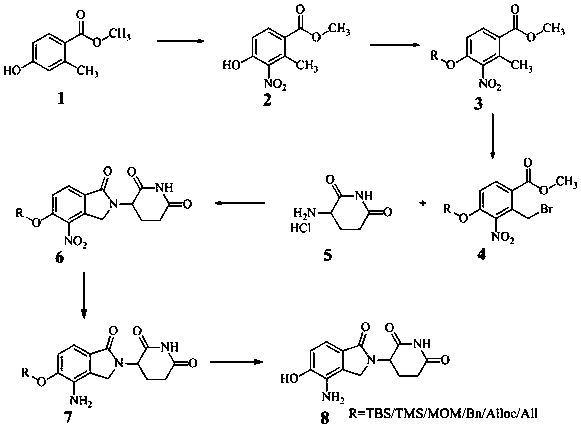
[0017] like figure 1 Shown, the preparation method of lenalidomide metabolite comprises the following steps:
[0018] Preparation of compound 2:
[0019] Dissolve 26 g of methyl 4-hydroxy-2-methylbenzoate in 80 mL of concentrated sulfuric acid, slowly add 15 mL of 65% concentrated nitric acid under ice bath, stir at room temperature for 2 hours, the reaction is complete, a large amount of solids precipitate out, suction filtration, The solid was washed with water, dried in a desiccator, and purified by a chromatographic column to obtain 15.2 g of compound 2 with a yield of 46%.
[0020] MS: 210[M-H - ]; 1H NMR: (400 MHz, DMSO-d6): δ 2.38 (s, 3 H), 3.81 (s, 3H), 6.9 (dd, 1 H), 7.9 (dd, 1 H), 11.8 (brs, 1 H)).
[0021] Preparation of compound 3
[0022] Dissolve 10 g of compound 2 in 120 mL of dimethylformamide, add 26 g of potassium carbonate and 8 g of chloromethyl methyl ether, and react at 65°C for 2 hours. Thin-layer chromatography shows that the reaction is complete. ...
Embodiment 2
[0034] like figure 1 Shown, the preparation method of lenalidomide metabolite comprises the following steps:
[0035] Preparation of Compound 2:
[0036] Dissolve 2.6 g of methyl 4-hydroxy-2-methylbenzoate in 8.0 mL of concentrated sulfuric acid, slowly add 1.5 mL of 95% concentrated nitric acid under ice bath, stir at room temperature for 1 hour, the reaction is complete, and solids are precipitated. After washing with water and drying in a desiccator, the solid was purified by chromatographic column to obtain 1.3 g of compound 2 with a yield of 43%.
[0037] Preparation of compound 3
[0038] Dissolve 1 g of compound 2 in 12 mL of dimethylformamide, add 2.5 g of potassium carbonate and 0.78 g of chloromethyl methyl ether, and react at 65°C for 2 hours. Thin layer chromatography shows that the reaction is complete. Extracted with ethyl acetate, washed with aqueous sodium chloride, dried over anhydrous sodium sulfate, and concentrated to obtain 1 g of compound 3 with a yiel...
Embodiment 3
[0048] like figure 1 Shown, the preparation method of lenalidomide metabolite comprises the following steps:
[0049] Preparation of compound 2:
[0050] Dissolve 13 g of methyl 4-hydroxy-2-methylbenzoate in 50 mL of concentrated sulfuric acid, slowly add 7 mL of 95% concentrated nitric acid under ice bath, stir at room temperature for 2 hours, the reaction is complete, and solids are precipitated, filter with suction 1. The solid was washed with water, dried in a desiccator, and purified with a chromatographic column to obtain 8 g of compound 2, with a yield of 47%. MS: 210[M-H - ]
[0051] Preparation of compound 3
[0052] Dissolve 5 g of compound 2 in 60 mL of dimethylformamide, add 13 g of potassium carbonate and 4 g of chloromethyl methyl ether, and react at 65°C for 2 hours. Thin-layer chromatography shows that the reaction is complete. The reaction solution is poured into water in ice and used Extracted with ethyl acetate, washed with aqueous sodium chloride, drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com