Self-repairing hydrogel based on di-selenium dynamic covalent bond and quadruple hydrogen bond
A technology of dynamic covalent bonds and quadruple hydrogen bonds is applied in the field of preparation of self-healing hydrogels to achieve the effects of excellent biocompatibility, designability of molecular structure, good mechanical properties and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
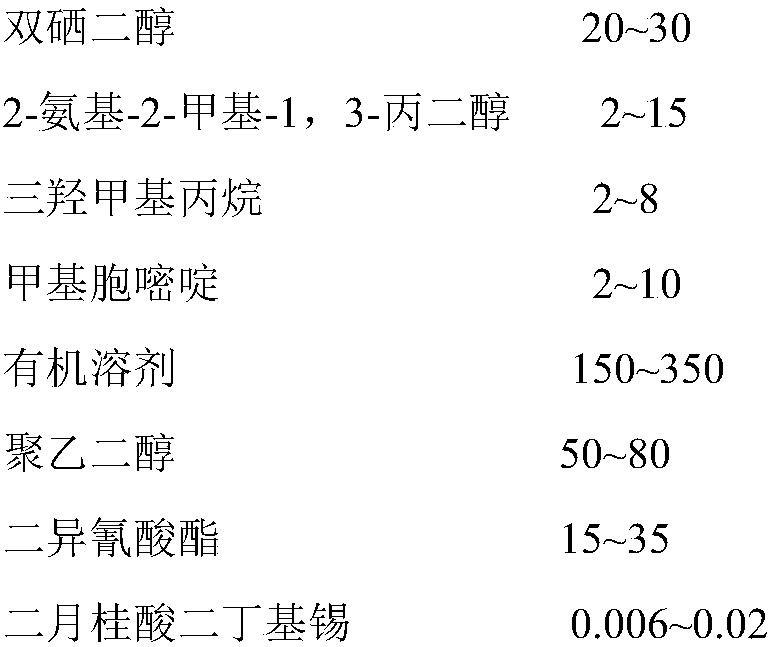
[0039]Example 1: At 110°C, use a rotary distillation device to distill PEG-1000 under reduced pressure for 3 hours under the condition of a vacuum of 0.09MPa to remove moisture; add 0.47g of 2-amino-4-hydroxyl to the reaction vessel -6-Methylpyrimidine and 0.86g IPDI were reacted at 120°C for 16 hours, then 20ml of n-pentane was added to precipitate the product, and the precipitate was filtered out using a sand core funnel, and washed three times with n-pentane, and the product was used Bake in a vacuum oven at 40°C and 0.09MPa for 2 hours to evaporate the solvent to get 1.65g of methylcytosine powder with isocyanate groups, namely UPy-NCO; at 30°C, 1.65g of UPy-NCO and 0.78g of 2- Amino-2-methyl-1,3-propanediol was refluxed in 30ml of chloroform for 5 hours, the product was centrifuged at 10,000r / min with a centrifuge, and the precipitated part was evaporated to dryness at 40°C and 0.09MPa to obtain UPy radical Chain extender 1.59g; Add 10g of dried PEG-1000 and 4.47g of IPDI...
Embodiment 2
[0040] Example 2: At 100°C, use a rotary distillation device to distill PEG-750 under reduced pressure for 4 hours under a vacuum of 0.09MPa to remove moisture; add 0.49g of 2-amino-4-hydroxyl to the reaction vessel -6-Methylpyrimidine and 0.73gIPDI were reacted at 120°C for 16 hours, then 20ml of n-pentane was added to precipitate the product, and the precipitate was filtered out with a sand core funnel and washed three times with n-pentane. Bake in an oven at 40°C and 0.09MPa for 2 hours to evaporate the solvent to dryness, and obtain 1.26g of methylcytosine powder with isocyanate groups, namely UPy-NCO; at 30°C, 1.26g of UPy-NCO and 0.76g of 2-amino- 2-Methyl-1,3-propanediol was refluxed in 30ml of chloroform for 6 hours, and the product was centrifuged at 10,000r / min in a centrifuge, and the precipitated part was evaporated to dryness at 40°C and 0.09MPa to obtain UPy-based chain extension Add 7.5g of dried PEG-750 and 4.51g of IPDI in sequence in a three-necked flask, kee...
Embodiment 3
[0041] Example 3: At 120°C, use a rotary distillation device to distill PEG-1000 under reduced pressure for 3 hours under a vacuum of 0.09MPa to remove moisture; add 0.46g of 2-amino-4-hydroxyl to the reaction vessel -6-Methylpyrimidine and 0.82g IPDI were reacted at 100°C for 20 hours, then 15ml of n-pentane was added to precipitate the product, and the precipitate was filtered out using a sand core funnel, and washed three times with n-pentane, and the product was used Bake in a vacuum oven at 40°C and 0.09MPa for 2 hours to evaporate the solvent to get 1.26g of methylcytosine powder with isocyanate groups, namely UPy-NCO; at 30°C, 1.26g of UPy-NCO and 0.69g of 2- Amino-2-methyl-1,3-propanediol was refluxed in 20ml of chloroform for 6 hours, the product was centrifuged at 10,000r / min with a centrifuge, and the precipitated part was evaporated to dryness at 40°C and 0.09MPa to obtain UPy radical Chain extender 1.29g; add 5g of dried PEG-1000 and 2.21g of IPDI in sequence to a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com