Rucaparib oral controlled-release and sustained-release pharmaceutical composition and uses thereof
A technology of recaprabib and controlled-release drugs, applied in the field of recaprabab pharmaceutical preparations, can solve the problems of drug loss, high cost, and high blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
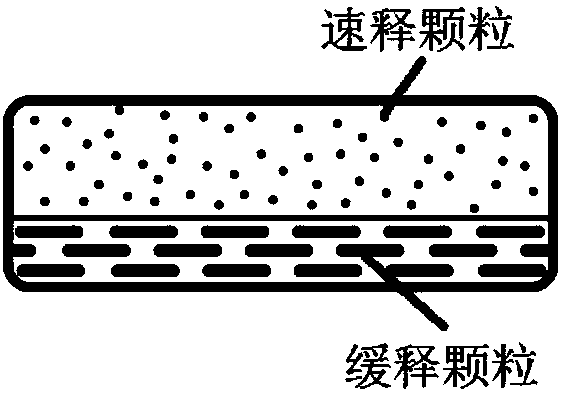
[0148] Example 1 Fast-Slow Double-Effect Release Matrix Tablet
[0149]
[0150] Immediate-release layer: Pass the prescribed amount of Recapabu, micronized silica gel and Soluplus through a 60-mesh sieve and mix with a three-dimensional mixer at 30 rpm for 25 minutes, then slowly add to the preheated melt extruder. The extrudate was collected and crushed through a 60-mesh sieve to obtain a solid dispersion of Recapabu. After the obtained solid dispersion of Recapabril is uniformly mixed with the prescription amount of other materials (disintegrant PVPP XL) and other auxiliary materials (mannitol and magnesium stearate), it is ready for tableting.
[0151] Sustained release layer: After passing the prescribed amount of Recapabu, PVP VA64 and micronized silica gel through a 60 mesh sieve and mixing them, slowly add them to the preheated melt extruder, collect the extrudates and crush them through 60 mesh The solid dispersion of Recapabub was obtained by sieving. The obtained solid...
Embodiment 1
[0156] Comparative Example 1 Immediate-release tablets
[0157] The prescription for immediate release tablets is as follows:
[0158]
[0159] The granular active ingredient ricapabu dextrocamphorsulfonate and the auxiliary materials microcrystalline cellulose PH101, sodium mannitol starch, colloidal silicon dioxide and magnesium stearate are mixed uniformly according to the prescription ratio, sieved, and placed in a three-dimensional multidirectional mixer Mix uniformly, use 98% ethanol as a binding solvent, sieving the granules, adding the prescribed amount of intergranular auxiliary materials and mixing them, and then press the tablet to obtain an immediate-release tablet prescription.
[0160] The dissolution rate is determined by the first method device of the dissolution test method (Chinese Pharmacopoeia 2010 Edition Two Appendix XC), at 37°C, with 900mL pH 2.0 release medium, rotating speed of 75 revolutions per minute, operating in accordance with the law, , 30, 45, 60, 7...
experiment Embodiment 1
[0163] The immediate-release tablets of Recapabu of Comparative Example 1 and the rapid-release dual-release matrix tablets of Example 1 were administered to full-bodied beagle dogs (n=3), respectively, and they were administered with 25 mL of water. After that, blood was taken at a predetermined time point. The blood sample was centrifuged at 4000 rpm for 10 min at 4°C, and the upper layer of plasma was taken for LC-MS blood concentration detection. The results are shown in Picture 10 .
[0164] Relative to C of immediate release tablet max (2750.3ng / mL) and AUC 0-h (19470h*ng / mL), C max Reduced to 1589.4ng / mL, a decrease of about 42%; AUC 0-h 20110h*ng / mL, change Picture 10 The results can still be seen, compared with the immediate-release tablets, the C max However, it can be maintained at a higher blood concentration for a long time, reducing the toxic and side effects caused by a sudden increase in the blood concentration, and at the same time, it can also extend the time fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com