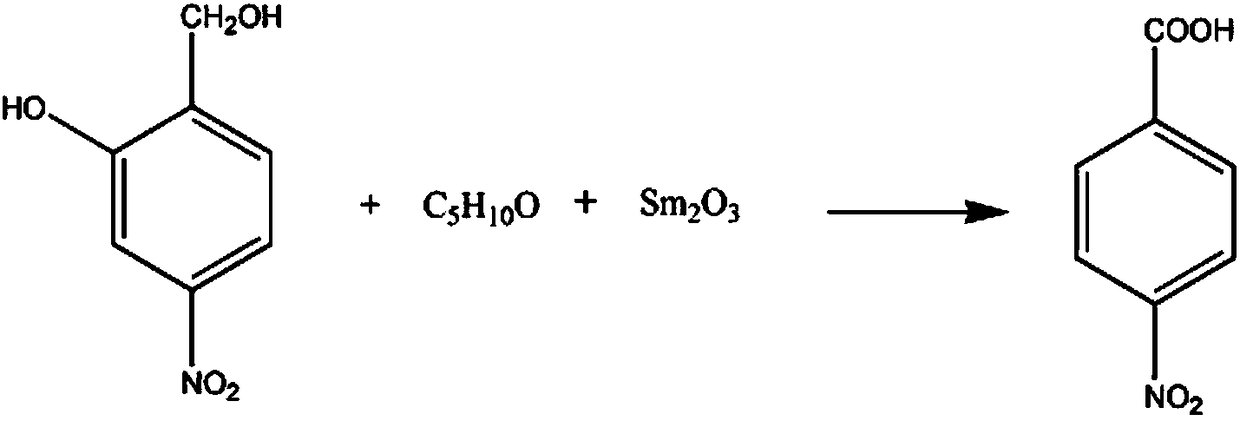Procaine hydrochloride drug intermediate p-nitrobenzoic acid synthesis method
A technology of procaine hydrochloride and p-nitrobenzoic acid, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of low safety factor in the production process, large environmental pollution, complex production process and the like , to achieve the effect of shortening the reaction time, increasing the reaction yield, and improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The synthetic method of procaine hydrochloride pharmaceutical intermediate p-nitrobenzoic acid comprises the steps:
[0018] A. Add 3mol of 4-nitro-6-hydroxybenzyl alcohol into the reaction vessel, raise the temperature of the solution to 46°C, control the stirring speed at 110rpm, and add 4mol of 3-methyl-2-butanone with a mass fraction of 80%. Solution, 2mol of samarium oxide powder, raise the temperature of the solution to 60°C;
[0019] B. Continue to react for 90 minutes, lower the solution temperature to 10°C, filter, wash 3 times with 30% potassium sulfate solution, 5 times with 10% oxalic acid solution, and 80% 2-chloro- Wash 6 times with 2-methylpropane solution, recrystallize in thiodiglycol solution with a mass fraction of 90%, and dehydrate with anhydrous calcium sulfate dehydrating agent to obtain 455.91 g of p-nitrobenzoic acid with a mass fraction of 91%.
Embodiment 2
[0021] The synthetic method of procaine hydrochloride pharmaceutical intermediate p-nitrobenzoic acid comprises the steps:
[0022] A. Add 3mol of 4-nitro-6-hydroxybenzyl alcohol to the reaction vessel, increase the solution temperature to 49°C, control the stirring speed at 120rpm, and add 4.5mol of 3-methyl-2-butanol with a mass fraction of 83%. Ketone solution, 2.5mol samarium oxide powder, raise the solution temperature to 63°C;
[0023] B. Continue to react for 110 minutes, lower the solution temperature to 15°C, filter, wash 4 times with 35% potassium sulfate solution, 6 times with 13% oxalic acid solution, and 82% 2-chloro-2 -Methyl propane solution washed 7 times, recrystallized in thiodiglycol solution with a mass fraction of 93%, and dehydrated with anhydrous potassium carbonate dehydrating agent to obtain 465.93 g of p-nitrobenzoic acid with a yield of 93%.
Embodiment 3
[0025] The synthetic method of procaine hydrochloride pharmaceutical intermediate p-nitrobenzoic acid comprises the steps:
[0026] A. Add 3mol of 4-nitro-6-hydroxybenzyl alcohol into the reaction vessel, raise the temperature of the solution to 55°C, control the stirring speed at 130rpm, and add 5mol of 3-methyl-2-butanol with a mass fraction of 86%. Ketone solution, 3mol samarium oxide powder, raise the solution temperature to 69°C;
[0027] B. Continue to react for 130min, lower the solution temperature to 18°C, filter, wash 5 times with 40% potassium sulfate solution, 6 times with 17% oxalic acid solution, and 85% 2-chloro - Washing 7 times with 2-methylpropane solution, recrystallizing in 96% thiodiglycol solution, and dehydrating with anhydrous calcium sulfate dehydrating agent to obtain 480.96 g of p-nitrobenzoic acid with a yield of 96%.
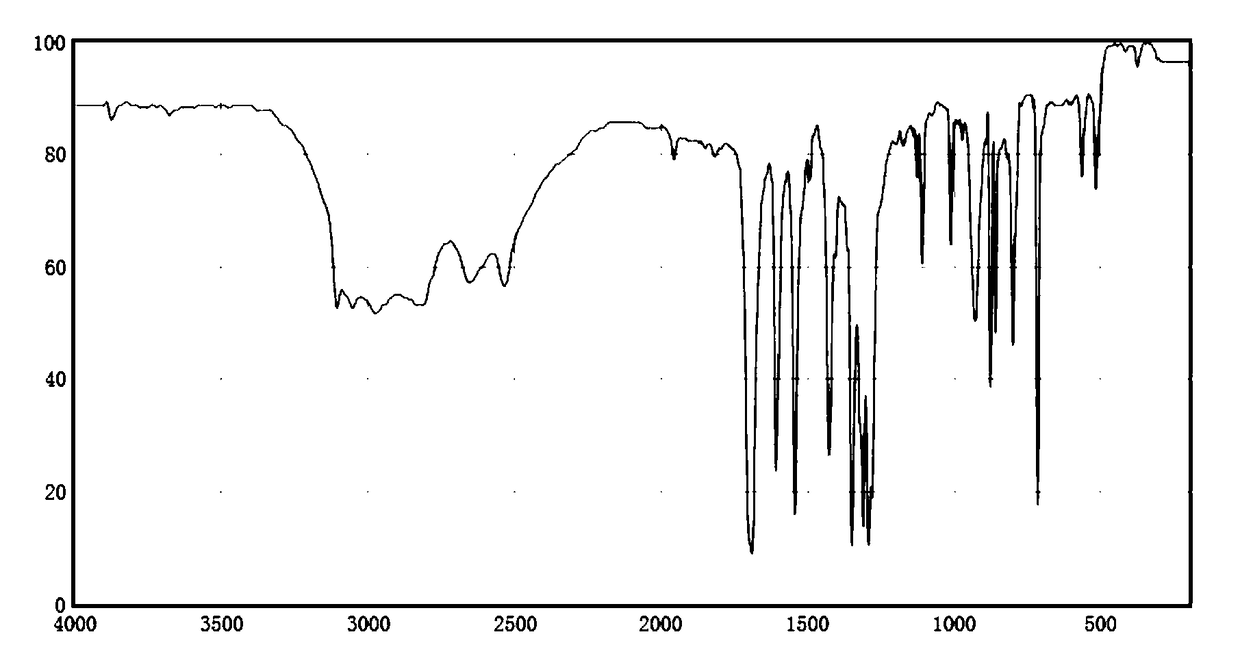
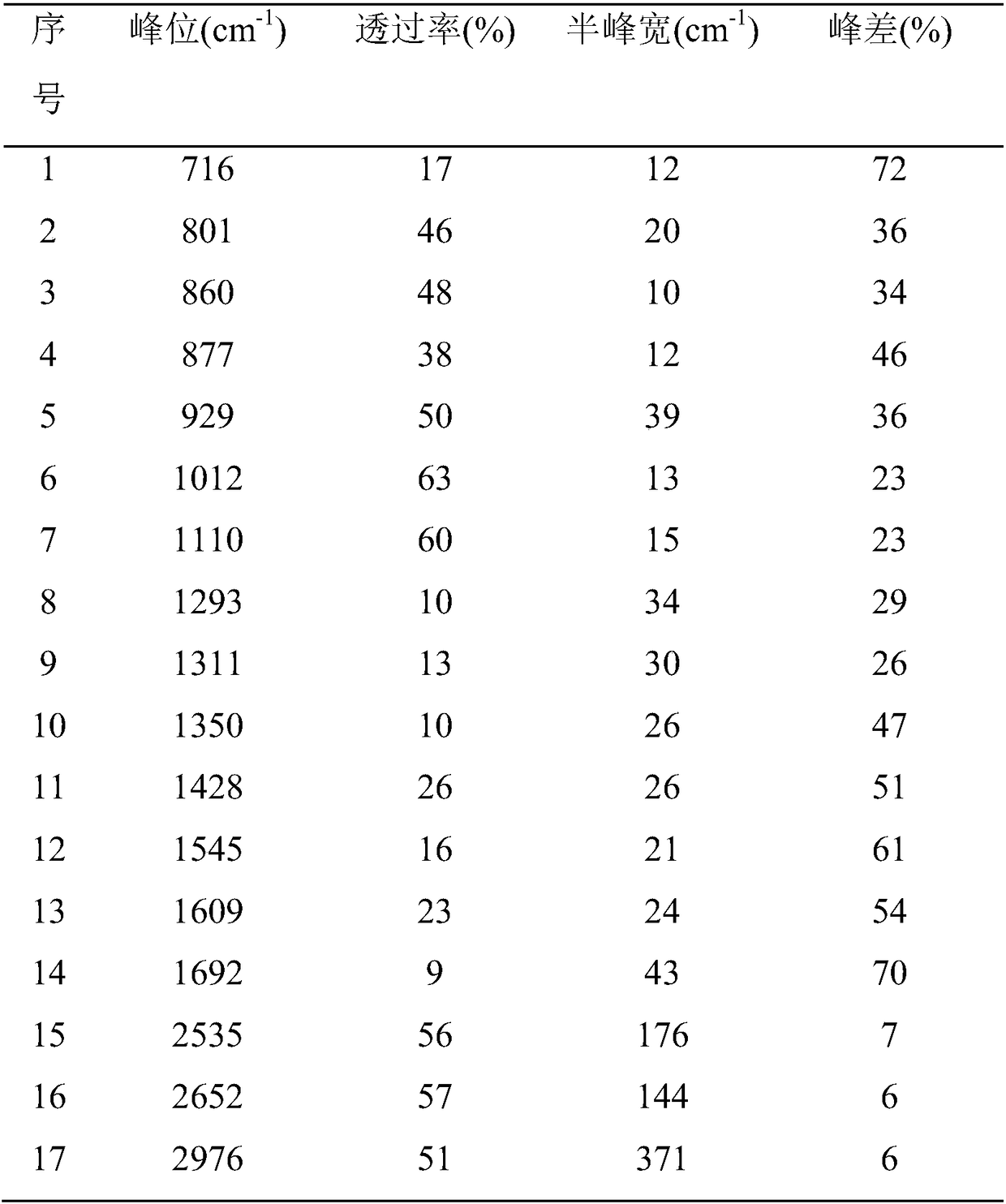
[0028] Finished product p-nitrobenzoic acid is done infrared analysis, gets infrared analysis spectrogram, as figure 1 shown. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com