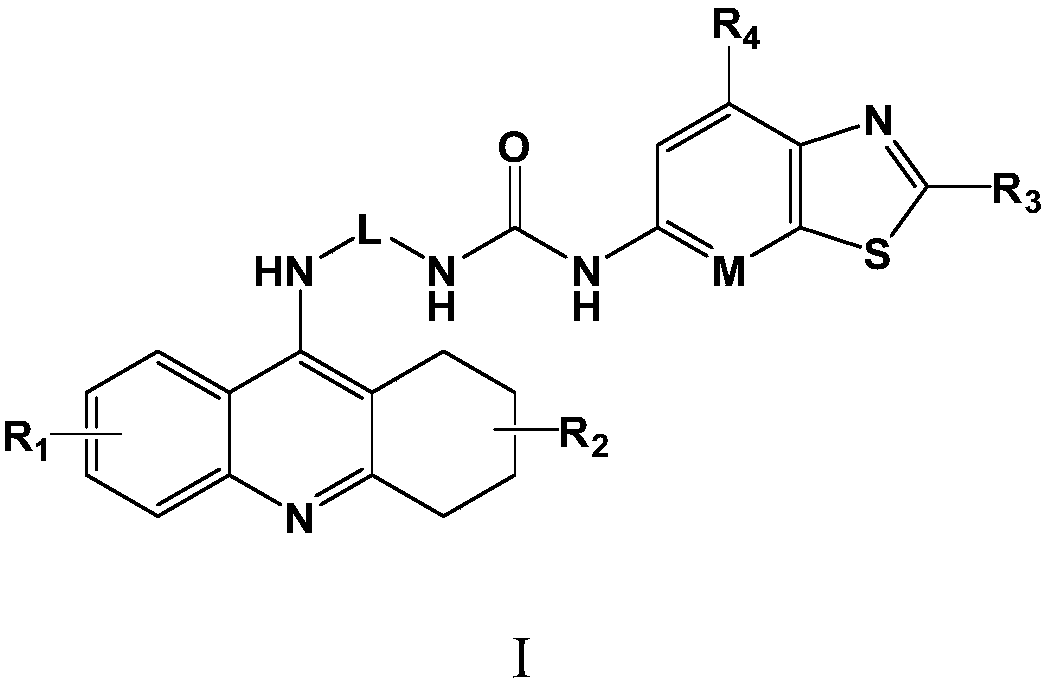
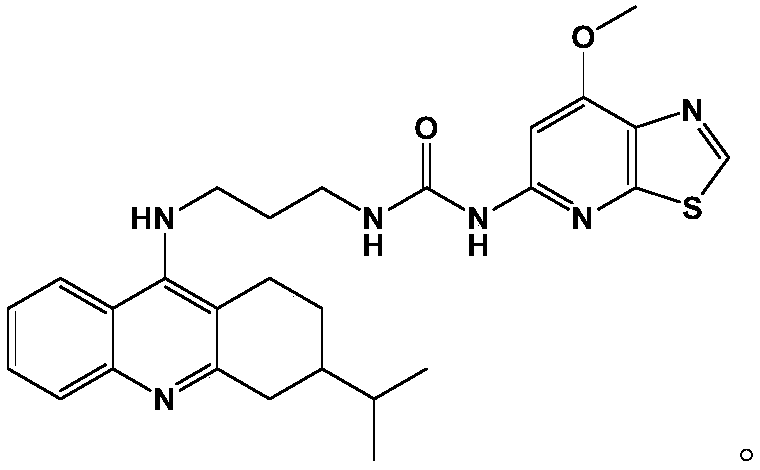
A kind of medicine for treating thyroiditis and preparation method thereof
A technology of pharmacy and compounds, applied in the field of medicine, can solve problems such as restricting iodine intake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
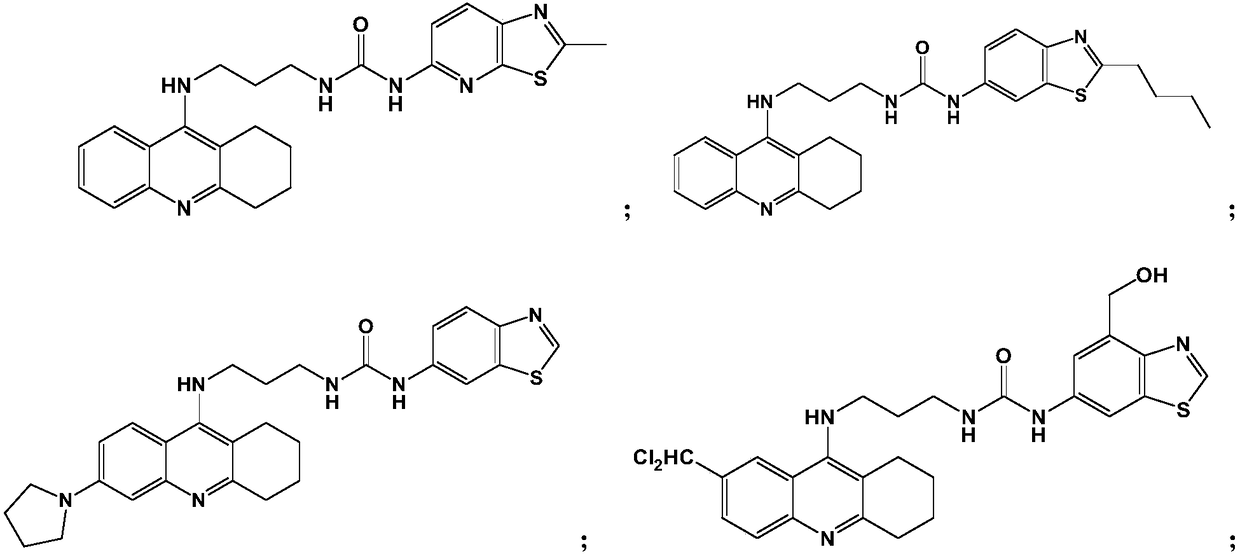
[0059] Example 1: 1-(2-methylthiazo[5,4-b]pyridin-5-yl)-3-(3-(1,2,3,4-tetrahydroacridin-9-ylamino) Propyl)urea (Compound 1)
[0060]
[0061] Heat the mixed solution of 4.34g of 9-chloro-1,2,3,4-tetrahydroacridine, 2.00g of 1,3-propylenediamine and 20ml of n-pentanol to 145°C to react under reflux 15h, cooled to room temperature, most of the solvent was removed by rotary evaporation, the residue was neutralized to weak alkalinity with saturated sodium bicarbonate solution and then washed with CH 2 Cl 2 Extract, combine the organic phases, wash the organic layer with water and saturated brine successively, dry over anhydrous sodium sulfate, filter and concentrate, the obtained residue is separated and purified by silica gel column chromatography (ethyl acetate:cyclohexane=1:1) to obtain the intermediate N 1 -(1,2,3,4-tetrahydroacridin-9-yl)propane-1,3-diamine 4.29g, yield 84.1%. ESI-MS[M+H] + = 256.17.
[0062] N under nitrogen 1 -(1,2,3,4-tetrahydroacridin-9-yl)propa...
Embodiment 2
[0066] Example 2: 1-(2-Butylbenzo[d]thiazol-6-yl)-3-(3-(1,2,3,4-tetrahydroacridin-9-ylamino)propyl) Urea (compound 2)
[0067]
[0068] N under nitrogen 1 -(1,2,3,4-tetrahydroacridin-9-yl)propane-1,3-diamine 2.55g was dissolved in 100ml of dichloromethane, stirred for 10 minutes, slowly added dropwise 2.29g of 2-butyl - A solution of 6-isocyanate benzo[d]thiazole dissolved in 100ml of dichloromethane, continue to stir for 20 minutes, slowly add dropwise a solution of 5ml triethylamine dissolved in 20ml of dichloromethane, after the dropwise addition, react at 30°C for 5h. The reaction solution was stirred with 200ml of water, the organic phase was washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and the concentrated solution was recrystallized with chloroform to obtain 3.82g of white crystals with a yield of 78.4%.
[0069] ESI-MS[M+H] + =488.24
[0070] Elemental analysis: theoretical value / measured value, C(68.96 / 68.89),...
Embodiment 3
[0072] Example 3: 1-(Benzo[d]thiazol-6-yl)-3-(3-(6-(pyrrolidin-1-yl)-1,2,3,4-tetrahydroacridine-9 -ylamino)propyl)urea (compound 3)
[0073]
[0074] Using a method similar to Example 1, replace 9-chloro-1,2,3,4-tetrahydroacridine with 6-(pyrrolidin-1-yl)-9-chloro-1,2,3,4-tetrahydroacridine Hydroacridine, substituting 6-isocyanate benzo[d]thiazole for 5-isocyanate-2-methylthiazo[5,4-b]pyridine afforded the title compound in 65.2% yield
[0075] ESI-MS[M+H] + =501.24[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com