A kind of preparation method of benzodiazepine * derivative
A compound, benzene sulfonic acid technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problem of low product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0069] The following examples are used to further describe the present invention, but these examples do not limit the scope of the present invention.
[0070] The experimental methods not indicating specific conditions in the examples of the present invention are generally in accordance with conventional conditions, or in accordance with the conditions suggested by raw material or commodity manufacturers. Reagents without specific sources indicated are conventional reagents purchased in the market.
Embodiment 1
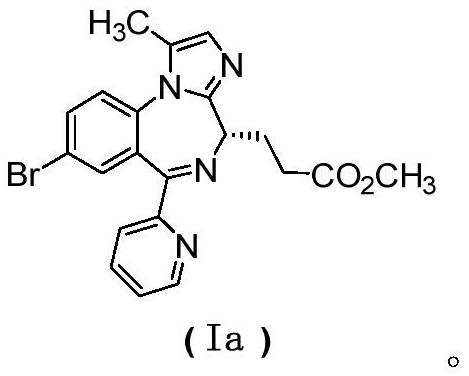
[0075] Example 1, (S)-3-(7-bromo-2-oxo-5-(pyridin-2-yl)-2,3-dihydro-1H-benzo[e][1,4]di Aza Preparation of -3-yl) methyl propionate
[0076]
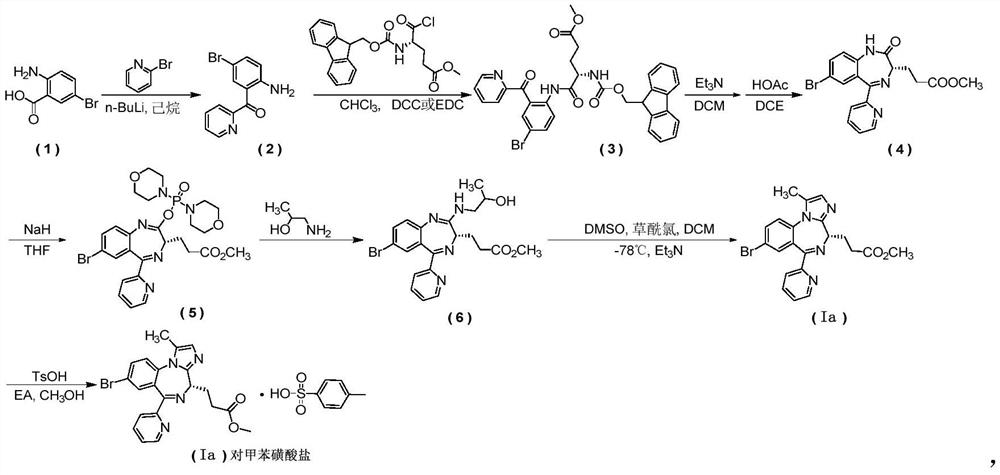
[0077] first step
[0078] (2-Amino-5-bromophenyl)(pyridin-2-yl)methanone
[0079] Add tetrahydrofuran (44mL) into the reaction flask, under nitrogen protection, add n-butyllithium (51mL) at -40°C, control the internal temperature not to exceed -20°C, lower the temperature to -40°C, and slowly add 2-bromopyridine ( 15.8g), after dropping, keep it warm for 1 hour, control the temperature below -40°C, add dropwise a solution of 2-amino-5-bromo-benzoic acid (6.7g) dissolved in (44mL) tetrahydrofuran, and naturally heat up to 0°C after dropping React for about 3 hours. Control the internal temperature below 10°C, slowly add saturated ammonium chloride solution (11mL) dropwise to terminate the reaction, then add water (50mL), let stand to separate the layers, separate the organic layer, and extract the aqueous layer with ethyl acetate...
Embodiment 2
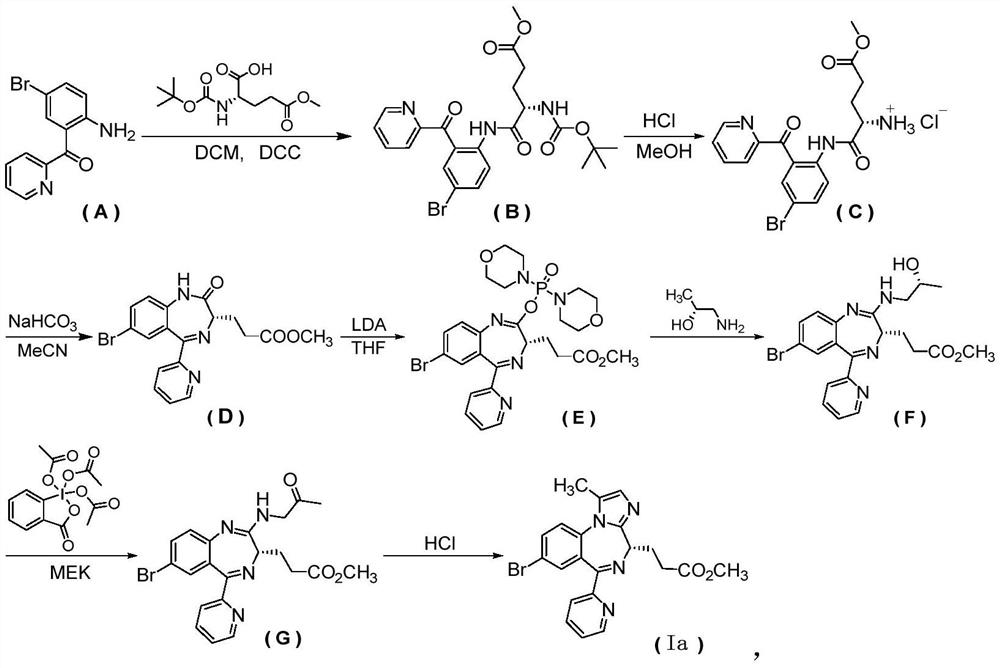
[0087] Example 2, (S)-3-(8-bromo-1-methyl-6-(pyridin-2-yl)-4H-benzo[f]imidazo[1,2-a][1,4 ] diazepine Preparation of -4-yl) methyl propionate
[0088]
[0089] first step
[0090] 3-((3S)-7-bromo-2-((2-hydroxypropyl)amino)-5-(pyridin-2-yl)-3H-benzo[e][1,4]diazepine -3-yl)methyl propionate
[0091] Under the protection of argon, add phosphorus oxychloride (1.2kg) into toluene (6.4kg) in the reaction bottle, stir to dissolve, cool to 5°C, drop morpholine (2.68kg) into it within 2h, and control the temperature Not exceeding 20°C. After dropping, react at room temperature for 3h. Filter the insoluble matter, wash it three times with toluene (1kg×3), combine the filtrate, concentrate under reduced pressure to an oily matter, add about (1.92kg) of toluene, heat to dissolve evenly, add petroleum ether (860g) under stirring (if there is insoluble matter , need to filter while it is hot), then add petroleum ether (3.48kg), cool to room temperature, filter, the filter cake is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com