Method for detecting nitrate nitrogen stable isotope in water
A technology of stable isotope and nitrate nitrogen, which is applied in the field of determination of stable isotope of nitrate nitrogen in water body, can solve the problems of unfavorable environment, long cultivation cycle of denitrifying bacteria, cumbersome operation, etc., and achieve the safety of operators, accurate and reliable measurement results, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
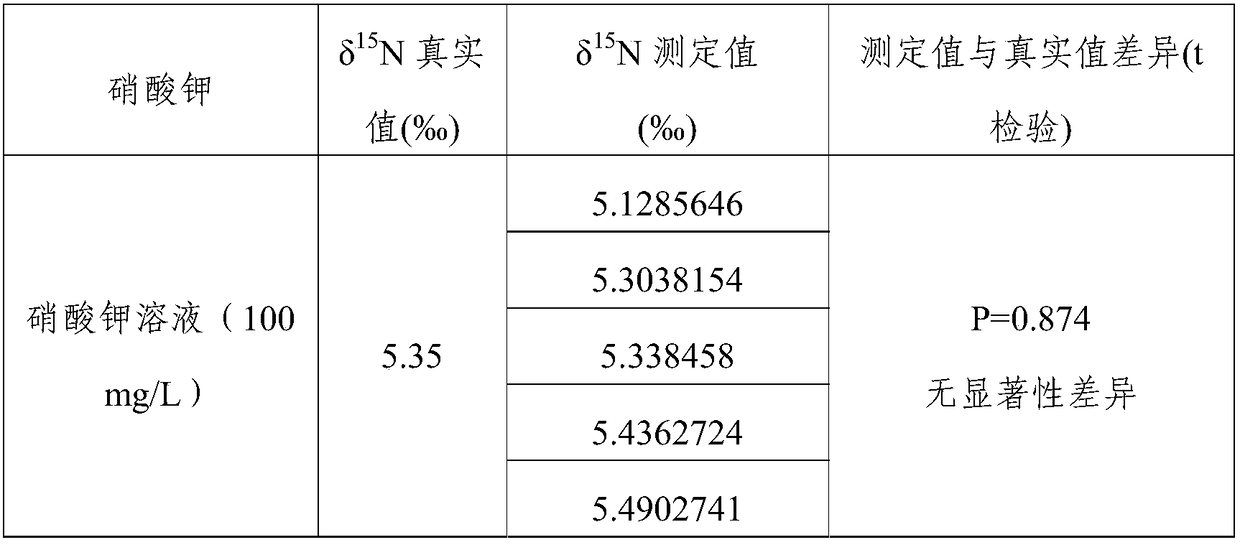
[0023] The present embodiment provides a kind of assay method of nitrogen stable isotope of nitrate in water, and the reagent and material involved mainly include: Potassium nitrate (precisely calibrated δ with elemental analyzer-stable isotope mass spectrometer) 15 N value is 5.35‰), benzene, concentrated sulfuric acid, nitrate isotope standard materials (USGS32, USGS34, USGS35).
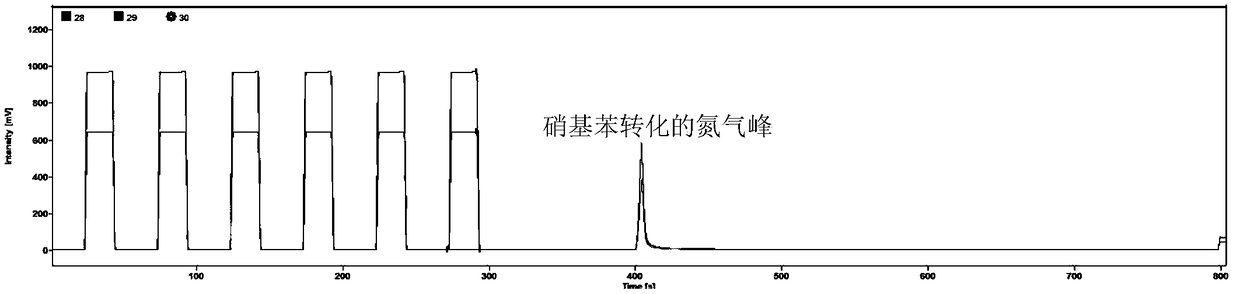
[0024] The method is specifically: the known δ 15 Potassium nitrate with an N value is prepared into a potassium nitrate solution with a concentration of 100 mg / L as the water body to be tested. Take 5 mL of the water body to be tested, pass through a 0.45 μm microporous membrane, add 1 times the volume of benzene and mix it in the resulting mixed solution. Slowly add 1.5 times the volume of concentrated sulfuric acid dropwise along the container wall, oscillate and mix for 2 minutes, then let the layers stand for 15 minutes, take the upper layer solution, remove water through anhydrous sodium sulf...
Embodiment 2
[0034] Compared with Example 1, the difference is only: the water body to be tested is 2 parts of environmental water bodies; the method described in Example 1 is used to detect the δ of 2 parts of environmental water bodies 15 The N values are 2.11±0.26‰ and 7.21±0.13‰, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com