A kind of zilpaterol compound veterinary drug composition and its preparation method and application
A composition and veterinary drug technology, applied in the direction of active ingredients of heterocyclic compounds, antibacterial drugs, pharmaceutical formulations, etc., can solve the problems of high cost of anti-CDV serum and monoclonal antibody treatment, complicated operation, and disease control, and achieve easy industrialization Simple application, simple operation, and long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
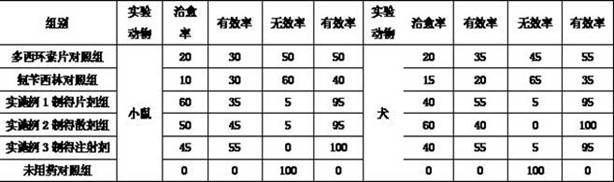
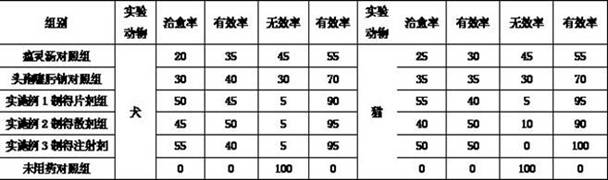
Image
Examples
Embodiment 1
[0038] 1. Prescription composition
[0039] Astragalus 1.5kg, Ephedra 1.5kg, Coptis 2kg, Shiwei 1.5kg, Bitter almond 1kg, Xanthium 0.5kg, Mint 0.5kg; Ribavirin 0.7kg, Zipaterol 0.45kg, Florfenicol 0.1 kg.
[0040] 2. Preparation method
[0041] Mint is extracted by steam distillation; astragalus, ephedra, berberine, Shiwei, bitter almonds, cocklebur seeds are crushed, passed through a 50-80 mesh sieve, added with ethanol for reflux extraction, and the extract is recovered from ethanol and concentrated to a relative density of Clear ointment I with a relative density of 1.05-1.15 at 60°C, set aside; mix the dregs extracted with alcohol and the volatile oil extracted from mint, add water to decoct, filter the extract, concentrate to 60°C with a relative density of 1.15-1.25 Clear cream II, set aside; mix clear cream I, clear cream II and peppermint volatile oil evenly to make a mixed clear cream; grind ribavirin, zilpaterol, and florfenicol into fine powder and mix with the pr...
Embodiment 2
[0045] 1. Prescription composition
[0046] Astragalus 2.5kg, Ephedra 2kg, Coptidis 1.5kg, Shiwei 1kg, Bitter almond 1.5kg, Xanthium 1.5kg, Mint 0.5kg; Ribavirin 0.5kg, Zipaterol 0.4kg, Florfenicol 0.3 kg.
[0047] 2. Preparation method
[0048] Mint adopts CO 2 Supercritical extraction method to extract volatile oil, extraction pressure 30MPa, temperature 55°C, CO2 Flow rate 25L / h, extraction 40min. ; Grind Astragalus, Ephedra, Coptidis, Shiwei, Bitter Almond, and Xanthium, pass through a 50-80 mesh sieve, add ethanol to reflux for extraction, recover ethanol from the extract, concentrate to a relative density of 1.05-1.15 at 60°C clear ointment I, set aside; mix the dregs extracted with alcohol and mint volatile oil, add water to decoct, filter the extract, and concentrate to clear ointment II with a relative density of 1.15-1.25 at 60°C, set aside; clear ointment II Paste I, Clear Paste II are uniformly mixed with peppermint volatile oil to obtain a mixed clear paste; R...
Embodiment 3
[0052] 1. Prescription composition
[0053] Astragalus 1kg, Ephedra 2.5kg, Coptidis 1kg, Shiwei 2kg, Bitter almond 0.5kg, Xanthium 1kg, Mint 1kg; Ribavirin 0.7kg, Zipaterol 0.3kg, Florfenicol 0.1kg.
[0054] 2. Preparation method
[0055] Mint adopts CO 2 Supercritical extraction method to extract volatile oil, extraction pressure 22MPa, temperature 40°C, CO 2 Flow rate 10L / h, extraction 90min. ; Grind Astragalus, Ephedra, Coptidis, Shiwei, Bitter Almond, and Xanthium, pass through a 50-80 mesh sieve, add ethanol to reflux for extraction, recover ethanol from the extract, concentrate to a relative density of 1.05-1.15 at 60°C clear ointment I, set aside; mix the dregs extracted with alcohol and mint volatile oil, add water to decoct, filter the extract, and concentrate to clear ointment II with a relative density of 1.15-1.25 at 60°C, set aside; clear ointment II Mix ointment I, ointment II and peppermint volatile oil evenly to prepare a mixed ointment; grind ribavirin, zi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com