A kind of industrial production method of octafluorocyclopentene
A technology for producing octafluorocyclopentene, which is applied in chemical instruments and methods, separation/purification of hydroxyl compounds, organic chemistry, etc., and can solve the problems of low product separation yield, many reaction steps, unsuitable raw materials, etc. Achieve the effect of high product purity, lower production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
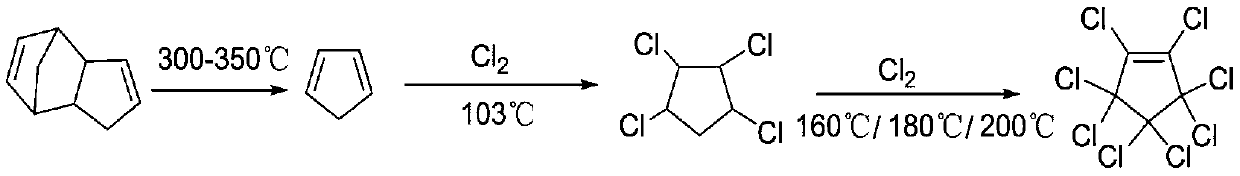
[0032] Embodiment 1: the synthetic route of octachlorocyclopentene in this example, by following reaction formula:
[0033]
[0034] Experimental steps:
[0035] Add 68g (1mol, 97%) liquid cyclopentene to a 250ml four-port reaction kettle equipped with mechanical stirring and reflux condensing device, and connect to an alkaline tail gas absorption device;
[0036] When the internal temperature of the system is 25°C, chlorine gas is started to flow into the kettle. At this time, the temperature of the system will rise, and the temperature at reflux is 45°C (the normal pressure boiling point of cyclopentene is 44.2°C), and the addition reaction of chlorine gas occurs, and there is no gas at the beginning. Production, chlorine gas is absorbed completely, and along with the carrying out of reaction, reflux stops, and the aeration rate of chlorine gas is optimal with 1g / min. At this time, a sample was taken for gas phase analysis, and the raw material cyclopentene was less than...
Embodiment 2
[0039] Embodiment 2: the synthetic route of octachlorocyclopentene in this example is the enlargement of example 1
[0040]
[0041] Experimental steps:
[0042] Add 680g (10mol, 97%) liquid cyclopentene to a 2L four-port reactor equipped with mechanical stirring and reflux condensing device, and connect to an alkaline tail gas absorption device;
[0043] Begin to feed chlorine gas in the kettle at room temperature, and the temperature of the system will rise at this time, and the temperature during reflux is 45° C. (the normal pressure boiling point of cyclopentene is 44.2° C.). Addition reaction occurs in feeding chlorine gas, so there is no gas production at the beginning. Chlorine is absorbed completely, and along with the carrying out of reaction, backflow stops, and the aeration rate of chlorine is optimal with 1g / min. At this time, a sample can be taken for gas phase analysis, and the raw material cyclopentene is <2%. Then slowly raise the temperature of the system...
Embodiment 3
[0046] Embodiment 3: the synthetic route of octafluorocyclopentene in this example, according to following reaction formula:
[0047]
[0048] Experimental steps:
[0049] Add 290g of anhydrous potassium fluoride (5mol, 99%, 10eq) and 1012g of sulfolane to a 2L four-port reactor equipped with mechanical stirring and rectification condensing device, and stir to raise the temperature to 140°C.
[0050]After mixing 172g (98%, 0.5mol) of self-made octachlorocyclopentene with 180g of sulfolane, dropwise added to the above system, the dropwise addition time is 2h, the temperature of the system rises slowly during the dropwise addition process, and the heating rate is about 5°C / h, After the dropwise addition, the system refluxed obviously, and the product was extracted from the top of the rectifying tower. The normal pressure boiling point of the product octafluorocyclopentene is 27°C.
[0051] When the product fraction decreases, continue to increase the temperature of the syst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com