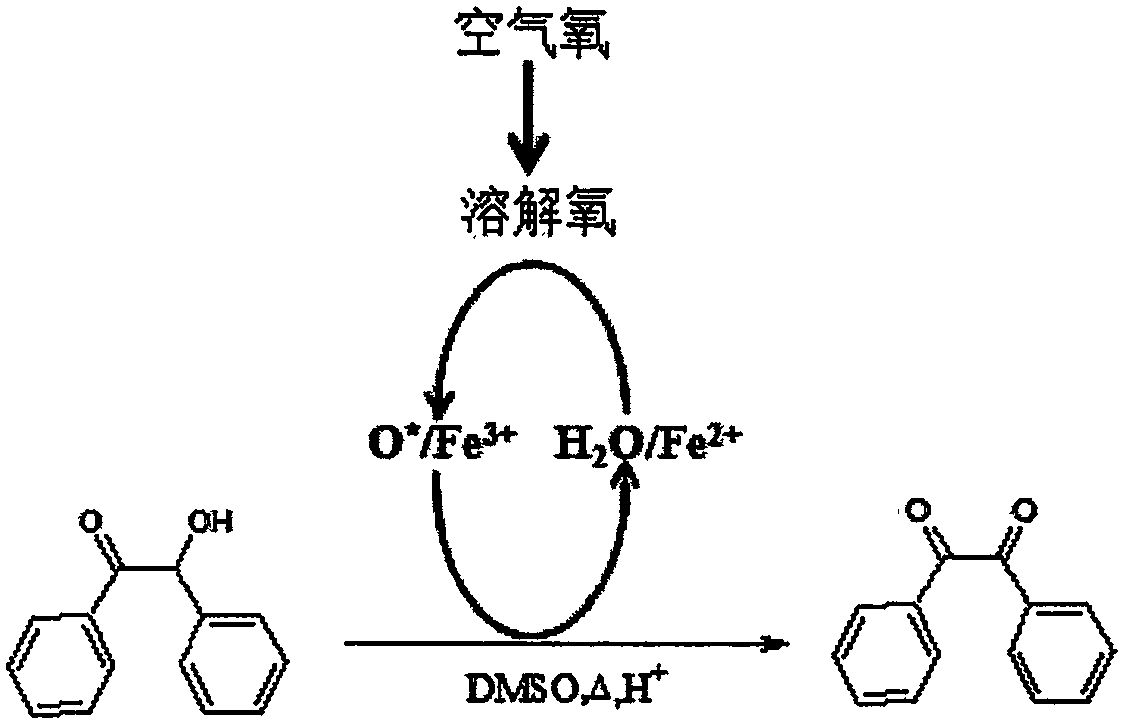
Method for preparing benzil by iron-catalyzed air oxidation of benzoin
A technology for oxidizing benzene and benzil, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc. Wide range, easy product solvent separation, no storage leakage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Build a reflux reaction device on a constant temperature water bath, adjust the reaction temperature to 80°C, adjust the hydrogen ion concentration to about 0.7mol / L with 33mL dimethyl sulfoxide (DMSO) with hydrochloric acid, add 2.1g benzoin and 0.2g catalyst FeNO 3 9H 2 O, reacted for 60min, after the reaction was finished, the reaction solution was poured into a beaker, after cooling to room temperature, an equal volume of distilled water was added, and after standing in an ice-water bath to precipitate solids, vacuum filtration was carried out, and the dried crude product was analyzed by high performance liquid chromatography for benzil The conversion rate reached 85.6%, and the crude ethanol was recrystallized to obtain yellow needle-like crystals with a measured melting point (mp) of 94.2-96.0°C.
Embodiment 2
[0025] Set up a reflux reaction device in an ultrasonic reactor, adjust the reaction temperature to 80°C, adjust the hydrogen ion concentration to about 1.2mol / L with 33mL dimethyl sulfoxide (DMSO) with hydrochloric acid, and add 2.1g benzoin and 0.2g catalyst FeNO 3 9H 2O, ultrasonic power 180W, reaction 60min, after the reaction, pour the reaction solution into a beaker, add an equal volume of distilled water after cooling to room temperature, leave it in an ice-water bath to precipitate solids, and carry out vacuum suction filtration, and dry the crude product by high performance liquid chromatography Analysis of benzil conversion rate reached 95.24%, good repeatability of synthetic reaction, RSD reached 0.57% (n=3), crude ethanol recrystallization obtained yellow needle-like crystals, measured melting point (mp) was 94.4-95.6 ° C, infrared spectrum ( FT-IR), ultraviolet-visible spectroscopy (UV-Vis), high-performance liquid chromatography-mass spectrometry coupled with ext...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com