Synthetic method of irbesartan
A synthetic method and methyl technology, applied in the field of pharmaceutical preparation, can solve the problems of life-threatening operators, difficulty in removing isomers, affecting the purity of the final product, etc., and achieve low-cost raw materials, convenient post-processing, and low equipment requirements. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
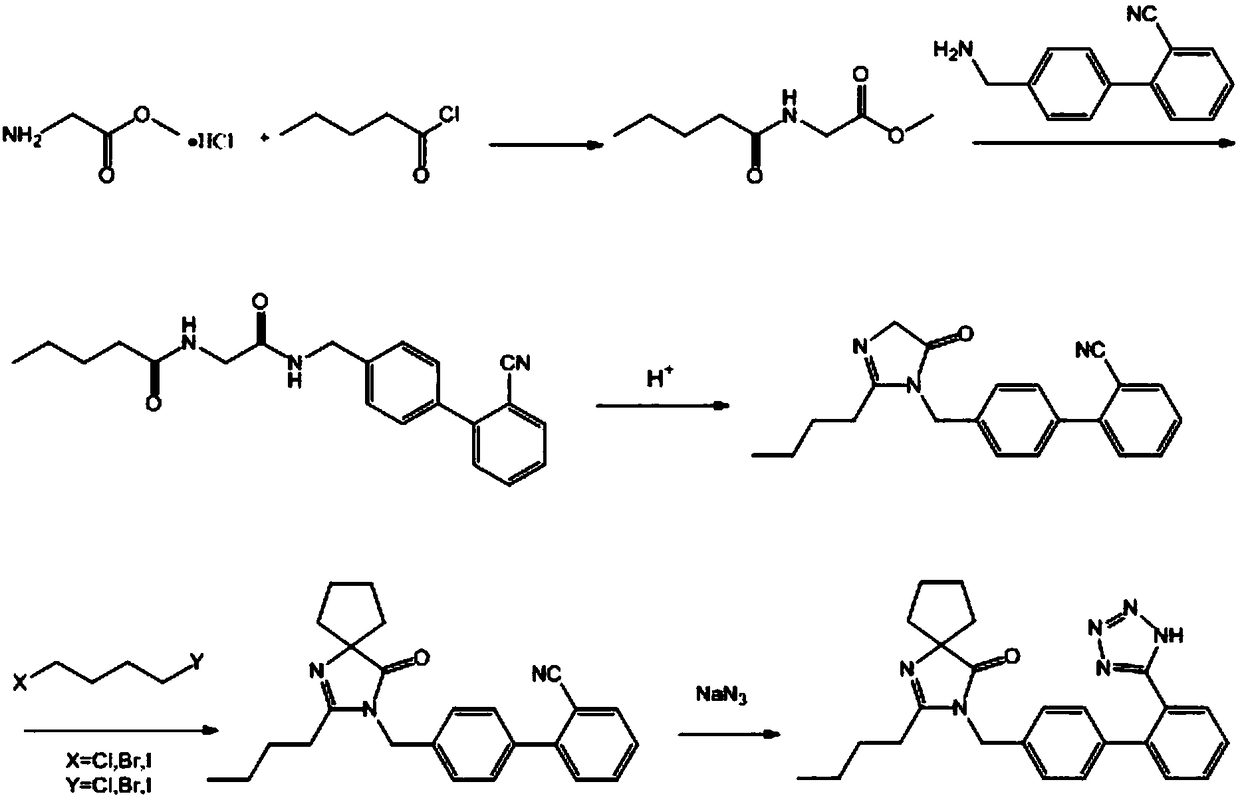
[0044] A synthetic method of irbesartan, comprising the following steps:
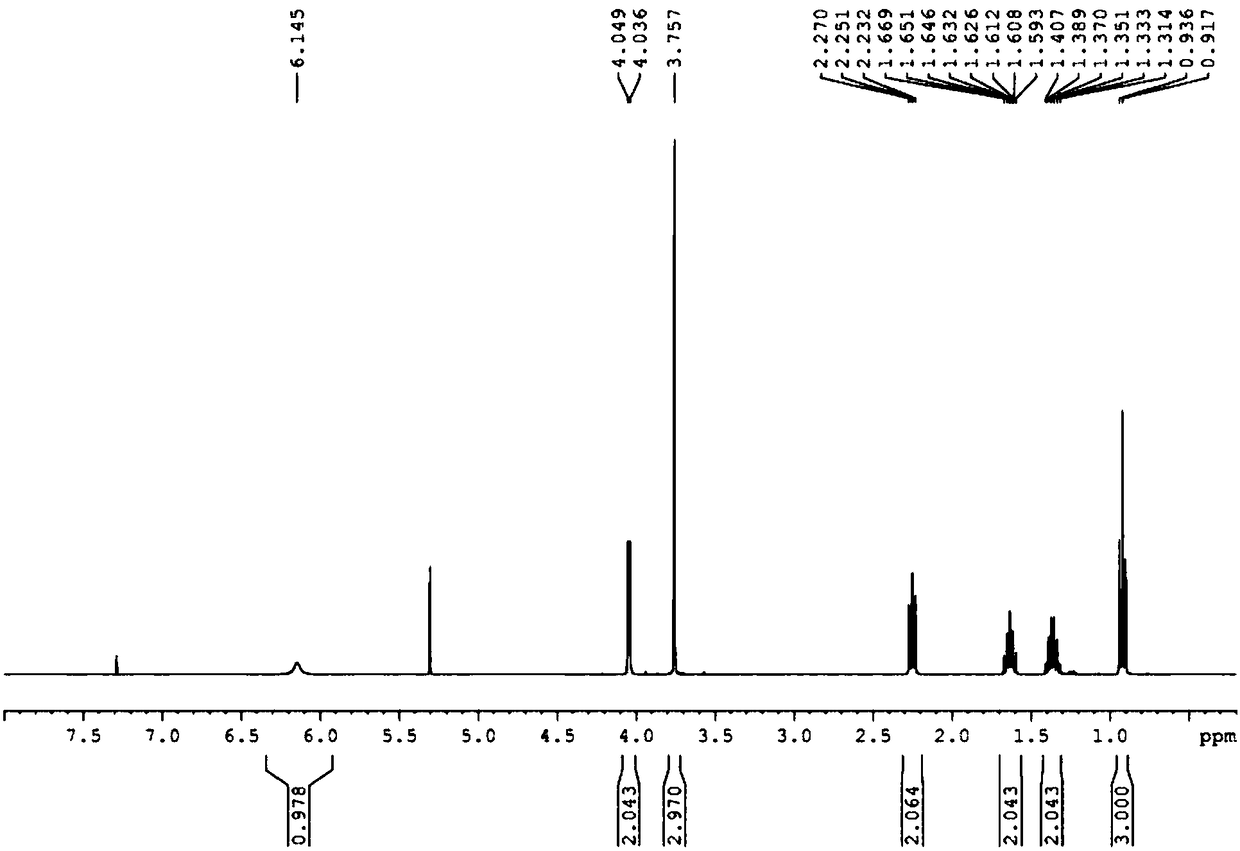
[0045] (1) Add glycine methyl ester hydrochloride (25.11g, 200mmol) and 200mL of dichloromethane in a 500mL single-necked bottle, cool to 0°C in a low-temperature bath, add triethylamine (34.7mL, 750mmol), and The reaction was stirred at °C for 0.5 h, then n-valeryl chloride (25.1 mL, 210 mmol) was slowly added dropwise, and reacted at 0 °C for 6 h. After the reaction, the reaction system was filtered, the filtrate was washed three times with saturated NaCl solution, the organic phase was dried with anhydrous sodium sulfate, filtered with suction, and the filtrate was evaporated to dryness to obtain 32.16 g of light yellow oily liquid N-n-pentanoyl glycine methyl ester, the yield 93.36%. The proton nuclear magnetic resonance spectrum of N-n-pentanoylglycine methyl ester is as attached figure 2 shown. 1HNMR (400MHz, Chloroform-d) δ6.02(s, 1H), 4.07(d, J=5.1Hz, 2H), 3.78(s, 2H), 2.27(t, J=7.6Hz, 2H), ...
Embodiment 2
[0051] A synthetic method of irbesartan, comprising the following steps:
[0052] (1) Add glycine methyl ester hydrochloride (25.11g, 200mmol) and 200mL of dichloromethane in a 500mL single-necked bottle, cool to -10°C in a low-temperature bath, add pyridine (31.64mL, 400mmol), and The reaction was stirred under low temperature for 0.5h, then n-valeryl chloride (23.9mL, 200mmol) was slowly added dropwise, and reacted at -10°C for 8h. After the reaction, the reaction system was filtered, the filtrate was washed three times with saturated NaCl solution, the organic phase was dried with anhydrous sodium sulfate, filtered with suction, and the filtrate was evaporated to dryness to obtain 30.34 g of light yellow oily liquid with a yield of 88.08%.
[0053] (2) Add 4'-aminomethyl-2-cyanobiphenyl (20.83g, 100mmol), N-n-pentanoyl glycine methyl ester (17.325g, 100mmol) and catalyst sodium ethylate (10.2 g, 150mmol), heated to reflux for 20h, after the reaction was over, most of the s...
Embodiment 3
[0058] A synthetic method of irbesartan, comprising the following steps:
[0059](1) Add glycine methyl ester hydrochloride (25.11g, 200mmol) and 200mL of dichloromethane in a 500mL single-necked bottle, cool to 0°C in a low-temperature bath, add triethylamine (70mL, 800mmol), and The reaction was stirred under low temperature for 0.5h, then n-valeryl chloride (28.7mL, 240mmol) was slowly added dropwise, and reacted at 20°C for 3h. After the reaction, the reaction system was filtered, the filtrate was washed three times with saturated NaCl solution, the organic phase was dried with anhydrous sodium sulfate, filtered with suction, and the filtrate was evaporated to dryness to obtain 33.21 g of a light yellow oily liquid with a yield of 96.41%.
[0060] (2) Add 4'-aminomethyl-2-cyanobiphenyl (20.83g, 100mmol), N-n-pentanoyl glycine methyl ester (27.72g, 160mmol) and catalyst potassium acetate (34.3 g, 350mmol), heated to reflux for 24h, after the reaction was over, most of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com