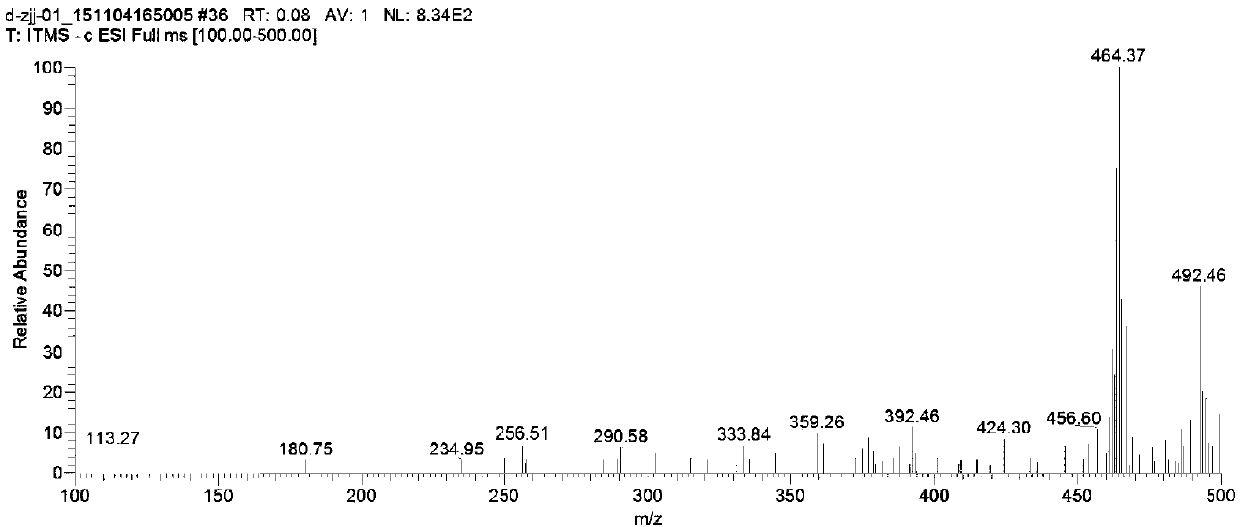
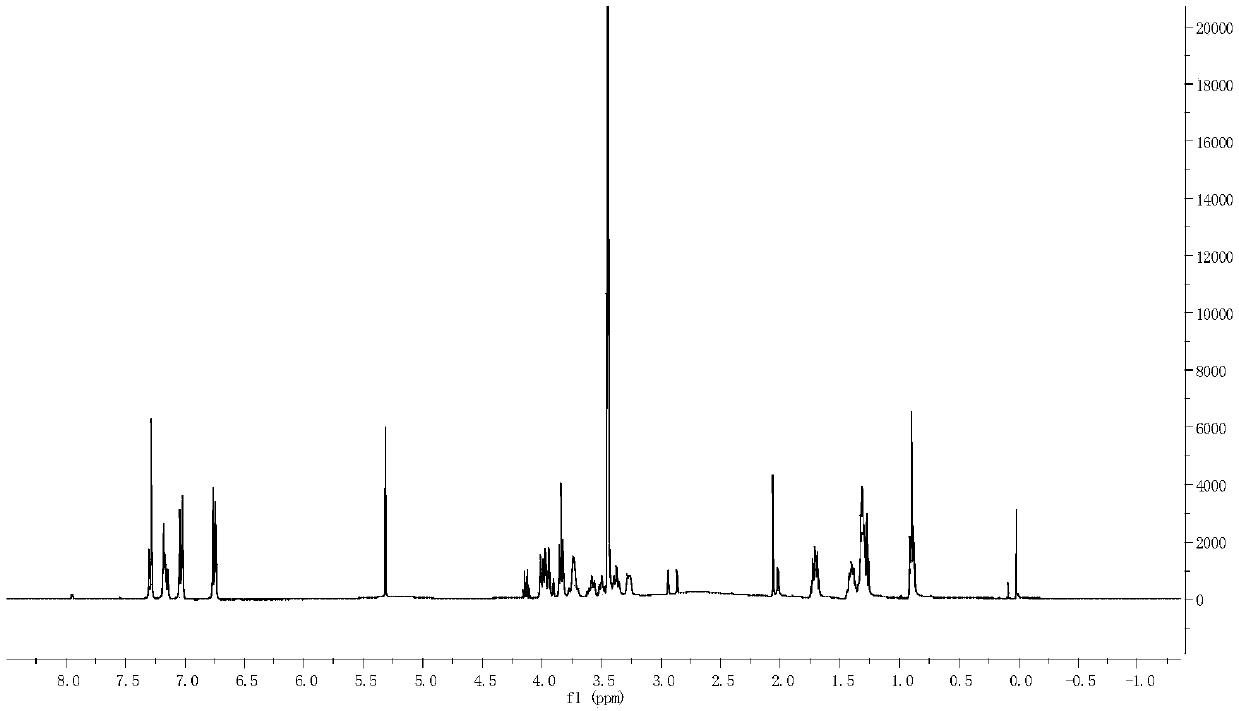
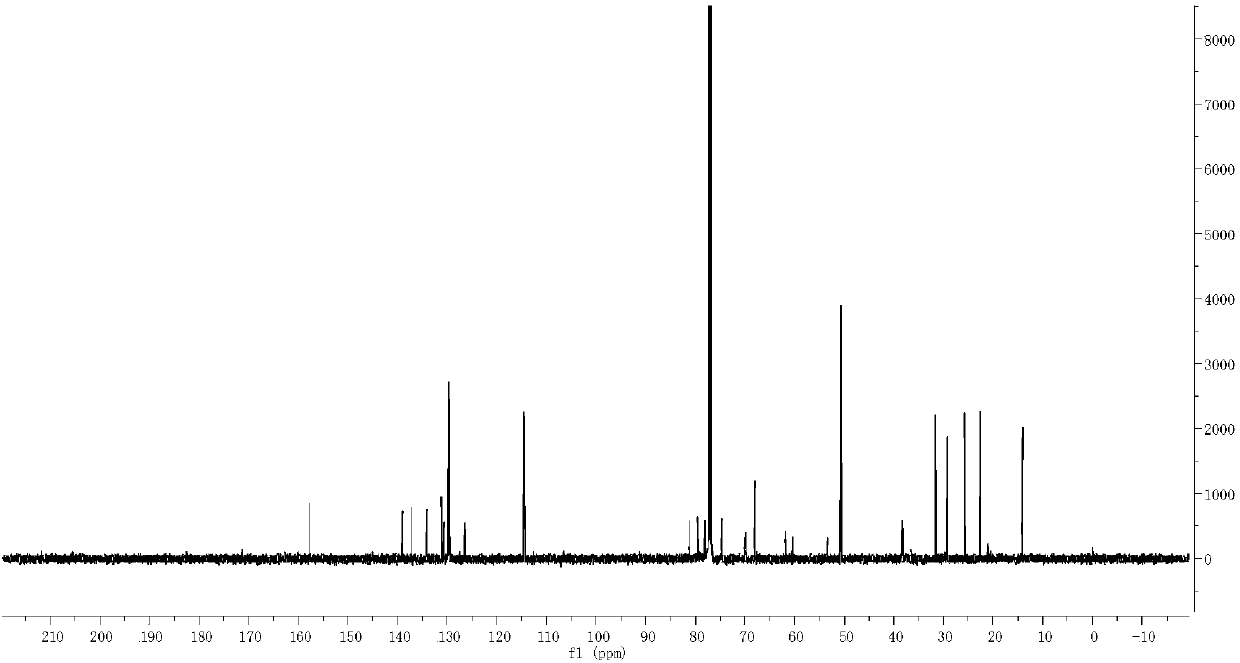
Carbon glucoside sodium glucose transport protein body 2 inhibitor
A technology of glucose transport and carbon glycosides, which is applied in the field of chemical medicine, can solve problems such as vomiting, short plasma half-life, and side effects, and achieve the effects of low side effects, strong hypoglycemic effect, and prolonged action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Synthesis of 5-bromo-2-chlorobenzoyl chloride (2)
[0093] 60.00 g (0.26 mol) of 5-bromo-2-chlorobenzoic acid (1) was added to 200 mL of dry dichloromethane, 1.5 mL (5.2 mol) of DMF was added dropwise, and four times were added to the reaction solution under ice-salt bath conditions. Slowly drop 32 mL (0.39 mmol) of oxalyl chloride into the mixture, and the temperature of the reaction solution is required to be between 0 and 5 °C. After the dropping is completed, the reaction solution is slowly raised to room temperature for 12 h. The reaction was monitored by thin-layer chromatography (TLC), until the reaction of the raw materials was completed, the solvent and oxalyl chloride were evaporated under reduced pressure, and the oxalyl chloride was evaporated three times with dichloromethane. m / z):255.1[M+H]+
Embodiment 2
[0095] Synthesis of 5-bromo-2-chloro-4'-methoxybenzophenone (3)
[0096] 34.10g (0.26mol) of anhydrous aluminum trichloride was added to dry 110mL of dichloromethane in three batches under ice-bath conditions, and 23mL (0.22mol) of anisole was slowly added dropwise to the reaction solution after stirring for 15min. The rate of addition makes the temperature of the reaction solution at 0 to 5° C. After 30 min, the dichloromethane (115 mL) solution of Intermediate 2 (66g) is slowly added dropwise to the reaction solution, and the rate of addition is controlled to keep the temperature of the reaction solution at 0 to 5° C. After dripping, the reaction was carried out in an ice bath for 4h. Raised to room temperature for 6h. After the reaction was completed, the reaction solution was slowly poured into 750 mL of ice water, stirred for 45 min, and the organic layer was separated, washed with 1 mol·L-1 aqueous sodium hydroxide solution, 2 mol·L-1 hydrochloric acid, and saturated br...
Embodiment 3
[0098] Synthesis of 5-bromo-2-chloro-4'-methoxydiphenylmethane (4)
[0099] Under stirring conditions, 5mL (0.31mol) of triethylsilane and 5.5g of 3 (0.17mol) were added to 20mL of a 1:2 mixture of dichloromethane and acetonitrile for reaction, and the temperature was controlled at 10 °C and slowly added 2.5mL of boron trifluoride ether solution, with the reaction proceeding, the reaction temperature was controlled not to exceed 20°C. The reaction was monitored by HPLC. If the reaction was not completed, the reaction was stirred overnight, and 0.5 mL of triethylsilane and 0.3 mL of boron trifluoride ether solution were added, and then the reaction temperature was raised to 50 °C and the reaction was stirred for 4 h. After cooling, the reaction was stopped with 5 mL of 7N KOH solution, the aqueous phase was extracted with dichloromethane (2×), the organic phases were combined, washed with 2N KOH and saturated brine (2×) successively, dried over anhydrous sodium sulfate, filtere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com