Application of 5,7-dibromo-8-(methoxymethoxy)-2-methylquinoline or its medicinal salt in treating breast cancer
A methoxymethoxy, methylquinoline technology, applied in the field of biomedicine, can solve the problems of patient invasion and metastasis, and the effect of antitumor drugs on cancer treatment effects, achieve good anti-breast cancer activity, and inhibit breast cancer cell invasion. , the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
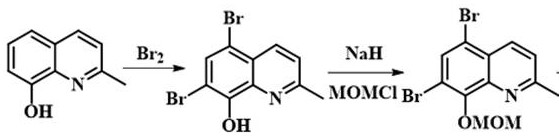
[0032] Embodiment 1: The preparation method of 5,7-dibromo-8-(methoxymethoxy)-2-methylquinoline
[0033] Weigh 2-methyl-8-hydroxyquinoline (3.18 g, 0.02 mol) and dissolve it in 30 mL of methanol in a 100 mL two-neck round bottom flask, then add sodium bicarbonate (3.18 g, 0.04 mol) and stir. Will Br 2 (3.2 mL, 0.06 mol) in methanol (10 mL) was slowly added to the bottle, the mixture was stirred for 30 min, and then Na 2 SO 3 (22 g, 0.17 mol) solid quenched the reaction, the mixture was filtered with suction, the filtrate was poured into water and stirred for 30 min, filtered with suction and dried in vacuo to obtain the white product 5,7-dibromo-2-methyl-8-hydroxyquinoline.
[0034] Take a tetrahydrofuran solution containing 5,7-dibromo-2-methyl-8-hydroxyquinoline (3.15 g, 0.01 mol) and slowly add it dropwise to NaH (1.0 g, 0.0417 mol) at a temperature of 0 °C In the tetrahydrofuran suspension, keep stirring at 0°C for 0.5 h after the dropwise addition, then slowly add ch...
Embodiment 2
[0035] Example 2: Culture and passage of breast cancer cells
[0036] The MCF-7 and MDA-MB231 breast cancer cells used in the experiment were cultured in Dulbecco's Modified Eagle Medium (DMEM) medium containing 10% fetal bovine serum, 100 U / ml penicillin and 100 μg / ml streptomycin, and the culture conditions were : 37°C, 5% CO 2 . Observe the growth status of the cells at any time. Generally, the cells can be subcultured when the density reaches 80% or above. The subculture method is as follows:
[0037] (1) Wash the cells once with phosphate buffered saline (PBS), then add an appropriate amount of trypsin to digest the cells until the cells become round and fall off.
[0038] (2) Neutralize the trypsin with 2 times the volume of fresh medium, and collect the cell suspension into a centrifuge tube.
[0039] (3) Centrifuge to obtain cell pellets at 800-1000 rpm for 5 minutes.
[0040] (4) Resuspend the cells with fresh medium and transfer them to a new culture dish at a rati...
Embodiment 3
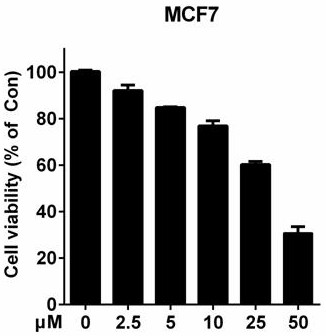
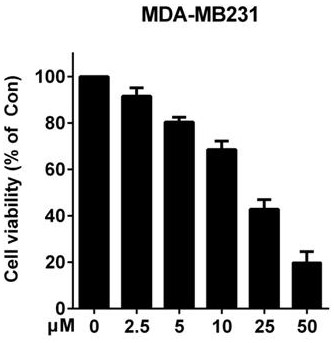
[0041] Example 3: Using the MTT method to screen quinoline derivatives with potential anti-breast cancer activity
[0042] The specific operation steps are:
[0043] (1) The cells in the logarithmic growth phase were digested and collected by trypsin, resuspended in DMEM medium containing 10% fetal bovine serum, mixed by repeated pipetting, and made into a single cell suspension.
[0044] (2) Cell counting: use a hemocytometer for cell counting with a configuration density of 8×10 4 cells / mL of cell suspension.
[0045] (3) Cell inoculation: Inoculate the cells into a 96-well plate with 100 μL of cell suspension per well.
[0046] (4) Cell culture: put the 96-well plate in CO 2 Incubator, at 37°C, 5% CO 2 Cells were cultured under saturated humidity and allowed to adhere to the wall overnight.
[0047] (5) Compound-treated cells: Prepare compounds with different concentration gradients (0 μM, 2.5 μM, 5 μM, 10 μM, 25 μM, 50 μM respectively), aspirate the medium in the 96-w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com