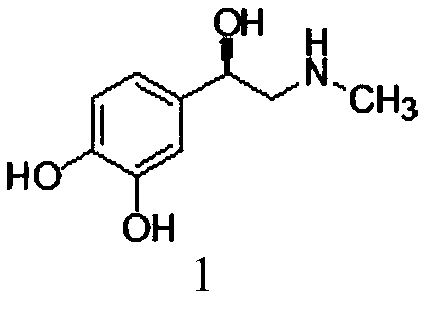
Preparation method of high-purity racemic epinephrine
A technology of adrenaline and racemization, which is applied in the field of medicine, can solve the problems of burden, waste liquid recovery and post-processing cost, etc., and achieve the effects of high recovery rate, cost saving and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 10 g of S-epinephrine (e.e.% is 36.8%) and 100 ml of purified water into a 250 ml round bottom flask, start stirring, and adjust the pH to 1.2 with hydrochloric acid. The air in the round bottom flask was replaced with nitrogen. Raise the temperature to 80±5°C. After heat preservation and stirring for 1 hour, cool down to 10±5°C. Add 0.5 g of activated carbon, use nitrogen to replace the air in the round bottom flask, and stir for 30 min. Filter and collect the filtrate. The filtrate was kept at 10±5°C, and the pH was adjusted to 9.0 with ammonia water. The solid was collected by filtration, rinsed with 10 ml of purified water first, and then rinsed with 10 ml of methanol. The solid was collected and dried to obtain 9.24 g of solid, with a weight yield of 92.4%.
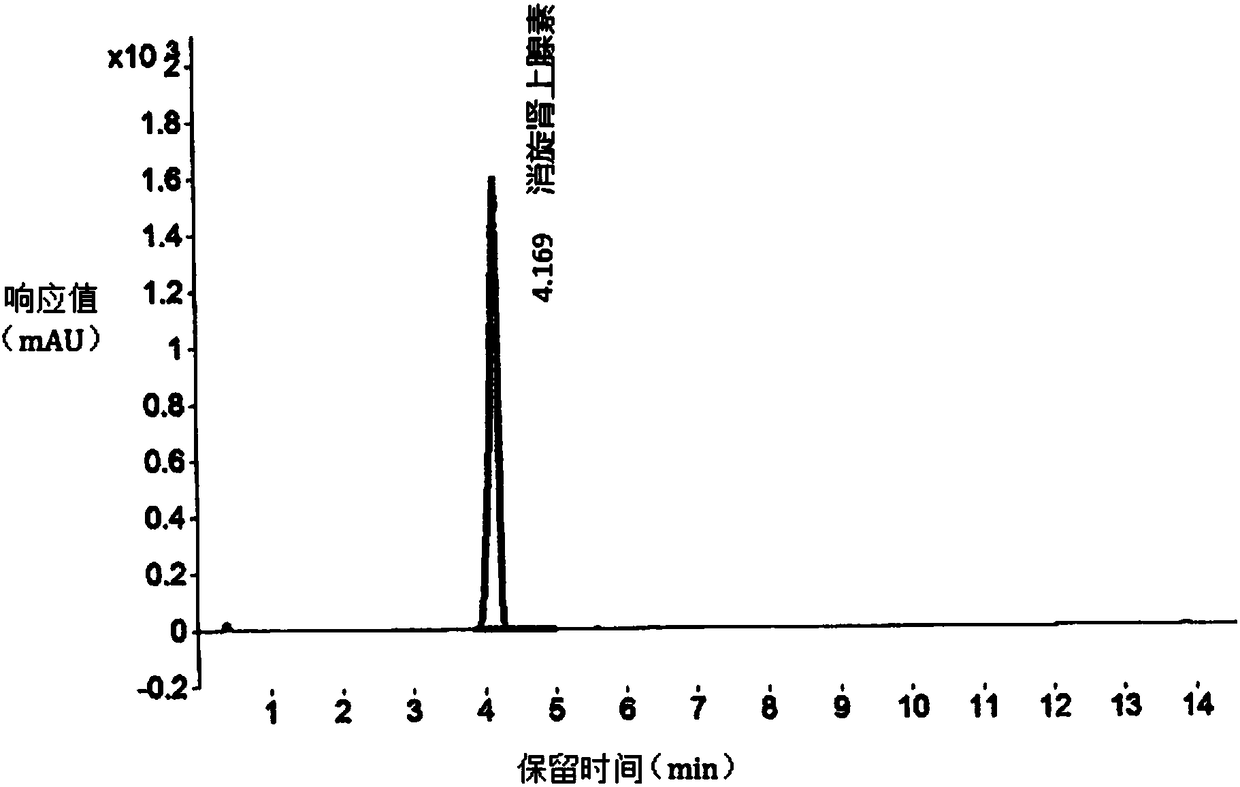
[0034] like figure 1 As shown, the retention time (RT) is 4.169min is racemic epinephrine, according to the integral peak area is 12845.69mAU*s, the total peak area is 12845.69mAU*s, the chromatograph...
Embodiment 2
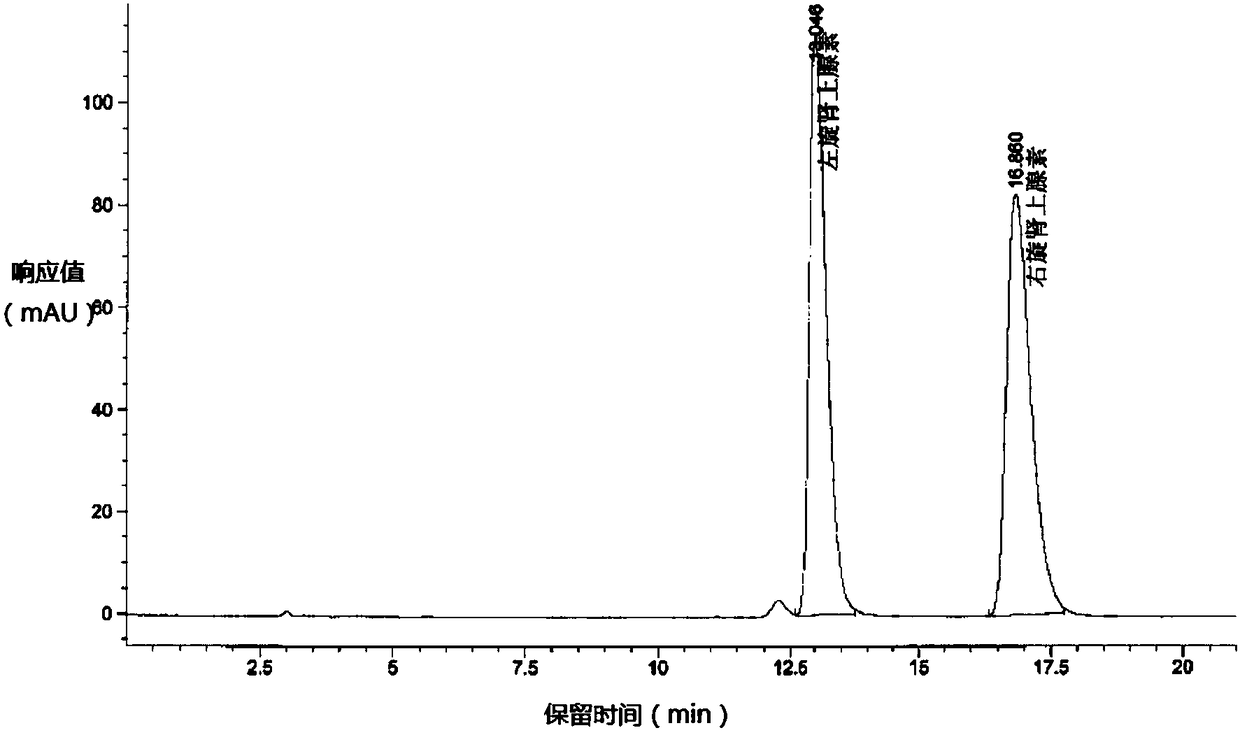
[0039] Add 10 g of S-epinephrine (e.e.% is 36.8%) and 100 ml of purified water into a 250 ml round bottom flask, start stirring, and adjust the pH to 1.0 with hydrochloric acid. The air in the round bottom flask was replaced with nitrogen. Raise the temperature to 80±5°C. After heat preservation and stirring for 1 hour, cool down to 10±5°C. Add 0.5 g of activated carbon, use nitrogen to replace the air in the round bottom flask, and stir for 30 min. Filter and collect the filtrate. The filtrate was kept at 10±5°C, and the pH was adjusted to 9.0 with ammonia water. The solid was collected by filtration, rinsed with 10 ml of purified water first, and then rinsed with 10 ml of methanol. The solid was collected and dried to obtain 9.31 g of solid, with a weight yield of 93.1%. Chromatographic purity 96.3%, e.e.% 0.49%.
Embodiment 3
[0041]Add 10 g of S-epinephrine (e.e.% is 36.8%) and 100 ml of purified water into a 250 ml round bottom flask, start stirring, and adjust the pH to 0.8 with hydrochloric acid. The air in the round bottom flask was replaced with nitrogen. Raise the temperature to 80±5°C. After heat preservation and stirring for 1 hour, cool down to 10±5°C. Add 0.5 g of activated carbon, use nitrogen to replace the air in the round bottom flask, and stir for 30 min. Filter and collect the filtrate. The filtrate was kept at 10±5°C, and the pH was adjusted to 9.0 with ammonia water. The solid was collected by filtration, rinsed with 10 ml of purified water first, and then rinsed with 10 ml of methanol. The solid was collected and dried to obtain 9.15 g of solid, with a weight yield of 91.5%. Chromatographic purity 97.8%, e.e.% 0.32%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Peak area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com