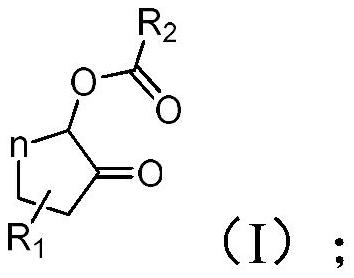
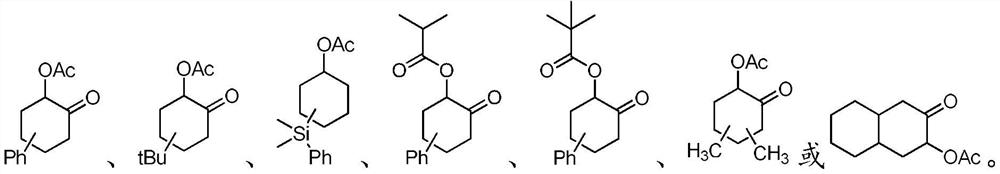
Method for synthesizing highly diastereoselective α-acyloxycyclic ketones
An acyl-oxidation cyclic ketone and diastereomeric technology is applied in the field of synthesizing α-acyl-oxidation cyclic ketone compounds with high diastereoselectivity, which can solve problems such as no stereoselectivity involved, and achieve controllable reaction products and chemical chemistry. High selectivity, easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
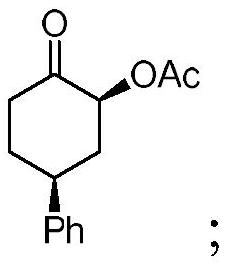
[0039] Dissolve 87.1mg (0.50mmol) of p-phenylcyclohexanone and 241.6mg (0.75mmol) of iodobenzenediacetic acid in 1.0mL of acetic acid, then add 185.1μL (3.00mmol) of boron trifluoride diethyl ether dropwise into the reaction system , after reacting at room temperature for 24 h, the reaction was quenched with 20 mL of saturated sodium bicarbonate and 5 mL of sodium thiosulfate (0.1 g / mL), extracted three times with dichloromethane, and the combined organic phases were concentrated under reduced pressure to obtain the crude product 1 . The crude product 1 was separated and purified by silica gel column chromatography (acetone:n-hexane=1:30) to obtain a white solid product 1 with a yield of 67%, dr=11.8:1.
[0040] Its characterization data are as follows:
[0041] Cis isomer, melting point: 90.1-95.1°C;
[0042] 1 H NMR (400MHz, CDCl 3 ):δ=7.35-7.23(m,5H),5.38(dd,J=12.8Hz,J=6.4Hz,1H),3.23(t,J=12.4Hz,1H),2.62-2.59(m,2H) ,2.49-2.44(m,1H),2.27-2.24(m,1H),2.17(s,3H)...
Embodiment 2
[0047]
[0048] Dissolve 87.1mg (0.50mmol) m-phenylcyclohexanone and 241.6mg (0.75mmol) iodobenzenediacetic acid in 1.0mL acetic acid, then add 185.1μL (3.00mmol) boron trifluoride diethyl ether dropwise into the reaction system , after reacting at room temperature for 48 h, the reaction was quenched with 20 mL of saturated sodium bicarbonate and 5 mL of sodium thiosulfate (0.1 g / mL), extracted three times with dichloromethane, and the combined organic phases were concentrated under reduced pressure to obtain the crude product 2 . The crude product 2 was separated and purified by silica gel column chromatography (acetone:n-hexane=1:30) to obtain a white solid product 2 with a yield of 47%, dr=6.1:1.
[0049] Its characterization data are as follows:
[0050] Trans isomer, melting point: 71.9-74.8°C;
[0051] 1 H NMR (400MHz, CDCl 3 ):δ=7.35-7.31(m,2H),7.25-7.20(m,3H),5.29(dd,J=6.4Hz,J=12.8Hz,1H),3.02-2.94(m,1H),2.70- 2.62(m,2H),2.40-2.35(m,1H),2.18(s,3H),2.14(m,1H),2.0...
Embodiment 3
[0056]
[0057] Dissolve 77.1 mg (0.50 mmol) of p-tert-butylcyclohexanone and 241.6 mg (0.75 mmol) of iodobenzenediacetic acid in 1.0 mL of acetic acid, then add 185.1 μL (3.00 mmol) of boron trifluoride diethyl ether dropwise into the reaction system, After reacting at room temperature for 24 h, the reaction was quenched with 20 mL of saturated sodium bicarbonate and 5 mL of sodium thiosulfate (0.1 g / mL), extracted three times with dichloromethane, and the combined organic phases were concentrated under reduced pressure to obtain crude product 3. The crude product 3 was separated and purified by silica gel column chromatography (acetone:n-hexane=1:30) to obtain a colorless and transparent liquid product 3 with a yield of 57%, dr=11.1:1.
[0058] Its characterization data are as follows:
[0059] cis isomer;
[0060] 1 H NMR (400MHz, CDCl 3 ):δ=5.23-5.18(m,1H),2.52-2.47(m,1H),2.43-2.34(m,1H),2.33-2.27(m,1H),2.15(s,3H),2.13-2.07 (m,1H),1.74-1.66(m,1H),1.57(q,J=12.4Hz,1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com