O-phenanthroline ruthenium complex photosensitive dye and its preparation method and use
A technology of o-phenanthroline ruthenium and photosensitizing dye, which is applied in the field of ruthenium complex photosensitizer to achieve strong therapeutic effect and increase the effect of treatment depth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The synthesis of embodiment 1. photosensitive molecule Ru-tmxf:
[0054]
[0055] (1) Synthesis of intermediate compound 3:
[0056] Ruthenium complex intermediate 1 (0.106g, 0.2mmol) was added to a 100mL single-necked flask containing 20mL ethanol and 10mL water, intermediate 2 (0.1g, 0.21mmol) was added, and refluxed for 24h under nitrogen protection. The solvent was evaporated under reduced pressure. Neutral alumina column separation (dichloromethane:methanol=15:1) gave an orange-red solid. Finally, the solid was dissolved in water, and saturated ammonium hexafluorophosphate solution was added to precipitate a red solid intermediate 3 (70%). 1 H NMR (500MHz, CD 3 SOCD 3 ), δ: 3.66(t, J=10Hz, 2H), 4.2(t, 10H, 2H), 7.00(d, J=10Hz, 2H), 7.38(d, J=10Hz, 1H), 7.49(t, J=8Hz, 1H), 7.58(d, J=10Hz, 2H), 7.79(m, 10H), 7.96(d, J=5Hz, 1H), 8.09(m, 4H); 8.39(d, J=10Hz , 4H), 8.77(m, 4H), 9.18(d, J=10Hz, 1H); ES-MS: m / z calcd for C 51 h 35 N 11 ORu 2+ [M–2PF 6 ] 2+ :...
Embodiment 2
[0060] Embodiment 2. Singlet oxygen performance test of photosensitive molecule Ru-tmxf
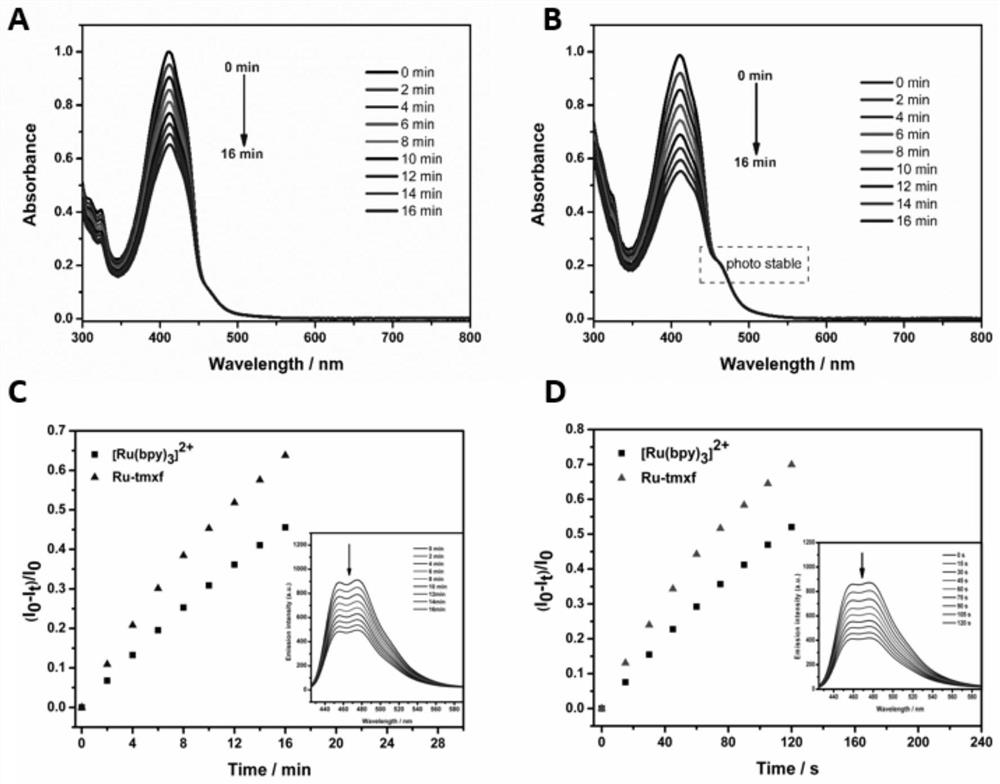
[0061] 10 μM reference [Ru(bpy) 3 ] 2+ Add the photosensitive molecule Ru-tmxf to the test quartz dish containing 3mL methanol solution, adjust the absorbance at 415nm to about 1 through the DPBF solution, place the dish at 450nm, 2mW / cm 2 The solution was irradiated under a xenon light source, and the absorption spectrum of the solution was recorded every two minutes. The test results are shown in figure 1 In Figures A and B, the absorption of the solution decays at a certain value as the irradiation time increases, indicating that the solution produces singlet oxygen under the irradiation of the light source of this wavelength, and as the irradiation time increases, the absorption spectrum of the solution The absorption at 460nm did not change, which to some extent shows that the photosensitive molecule Ru-tmxf has good photostability. Similarly, 10 μM of photosensitive molecule Ru...
Embodiment 3
[0062] Example 3. Target competition experiment
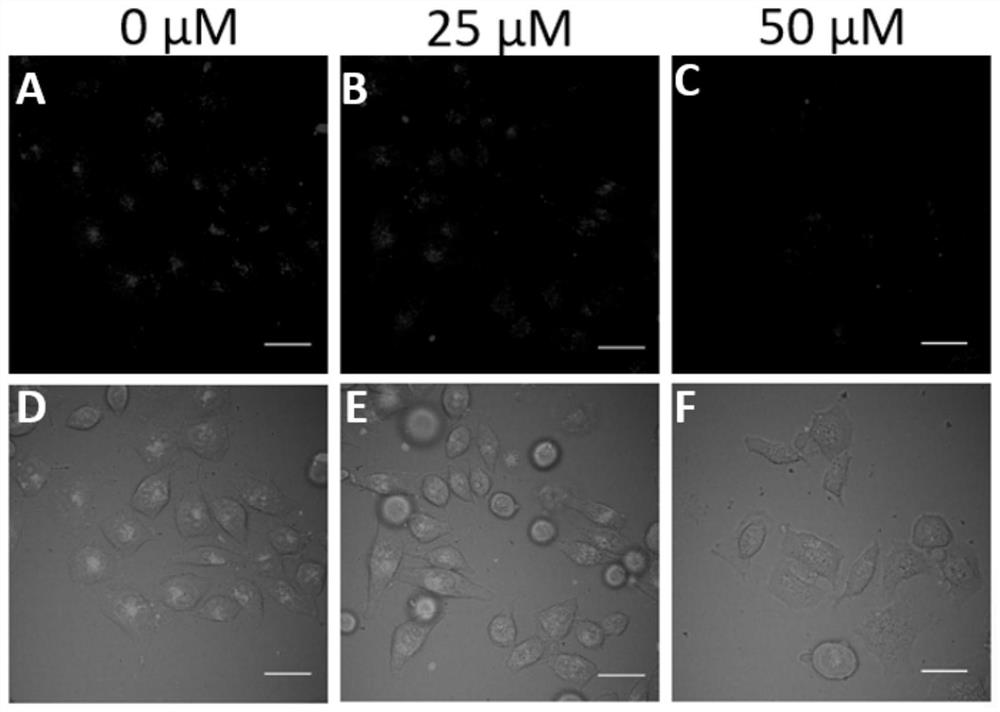
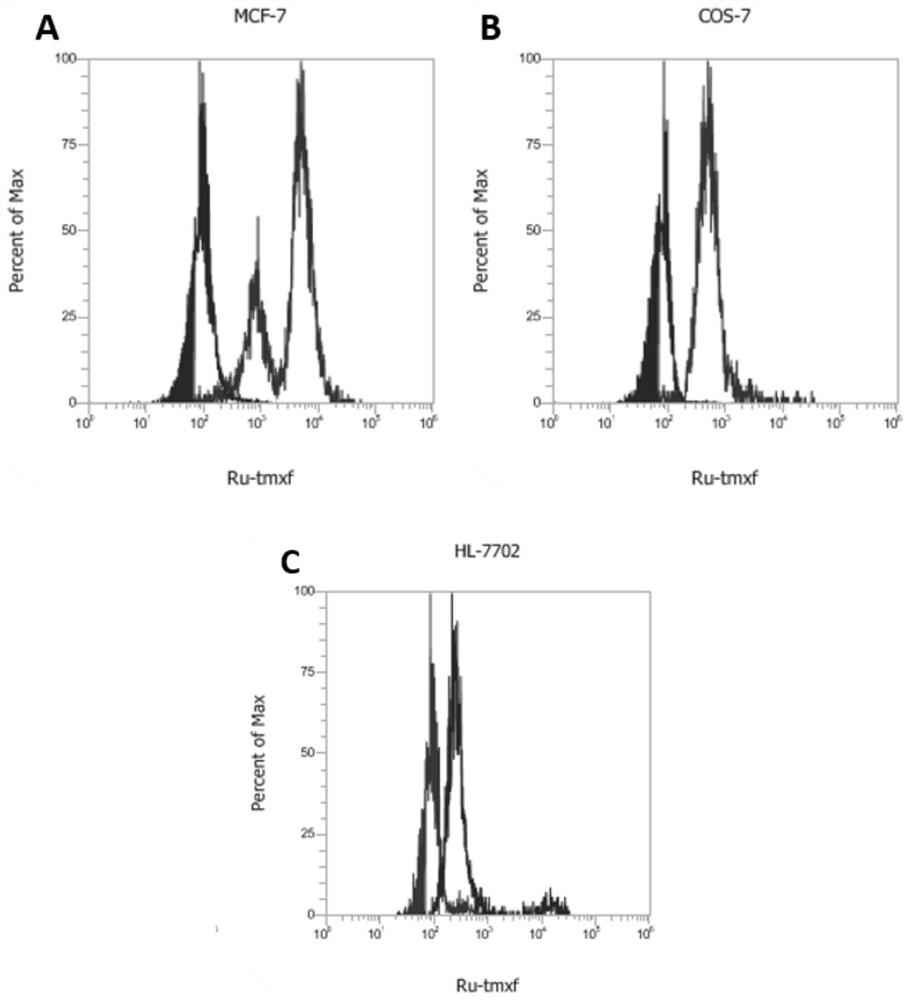
[0063] MCF-7 and COS-7 cells were cultured in DMEM (invitrogen) with 10% FCS (invitrogen). 17β-estradiol was used as a competitive inhibitor of Ru-tmxf entry into MCF-7 cells. 0, 25, and 50 μM 17β-estradiol were pre-added to the dish containing MCF-7 cells for 24 hours, and then 3 μM compound Ru-tmxf was added and incubated for 2 hours, and then laser confocal imaging was used. The excitation wavelength of the photosensitive molecule is 488nm, and the acceptance wavelength range is 570nm-620nm. from figure 2 It can be seen in A, B and C (D, E and F are the mixed images of bright field and fluorescent field) that the cellular uptake of competitive inhibitors is reduced, and the inhibitory strength increases with the increase of concentration .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com