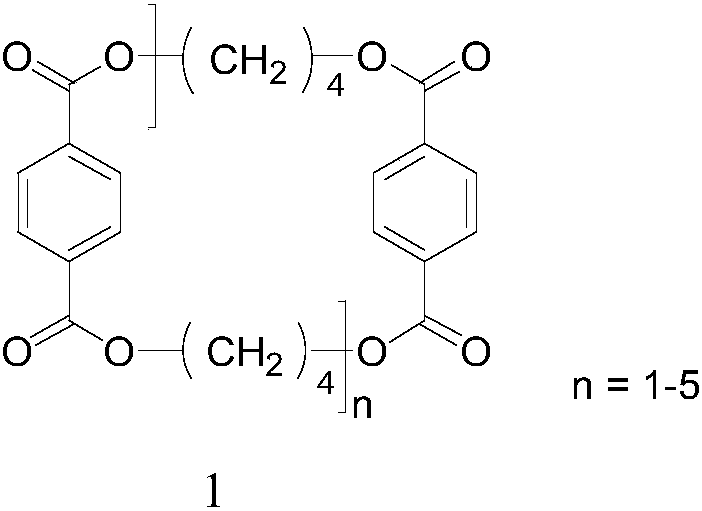
Preparation method of cyclic tetrapolybutylene terephthalate
A technology of butylene phthalate and phthalic acid chloride, applied in the field of compound preparation, can solve the problems of low yield, complicated operation, high cost and the like, and achieve the effects of high yield, high product quality and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
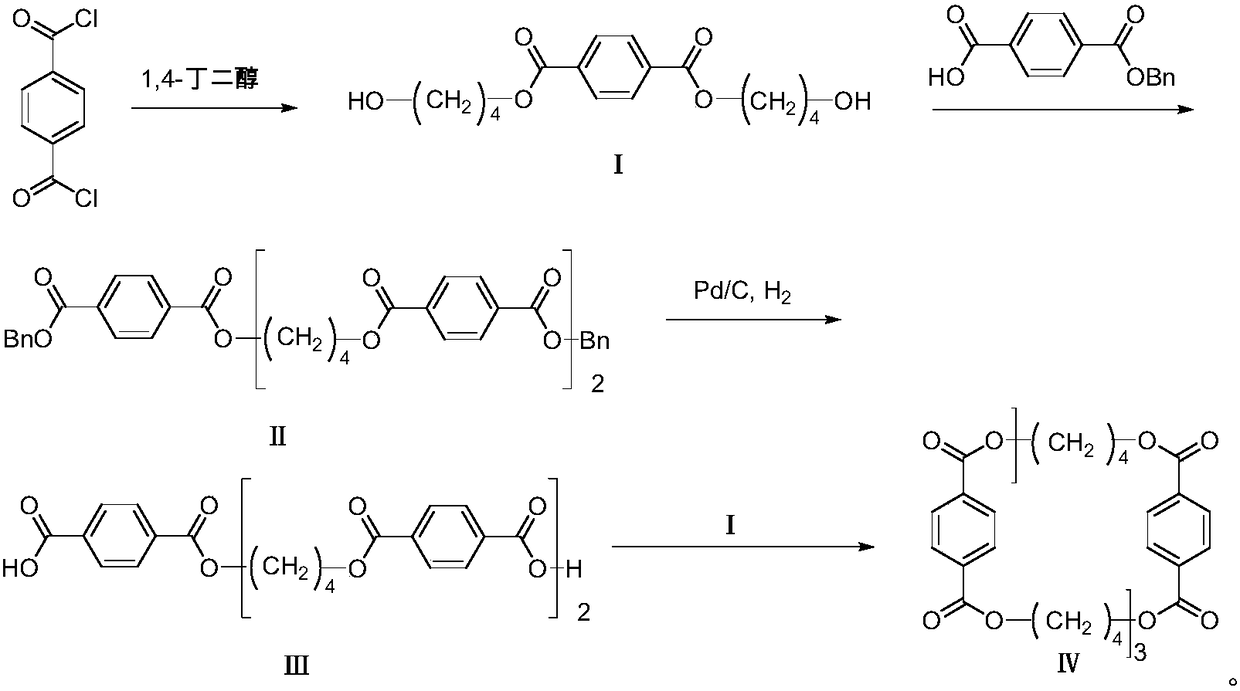
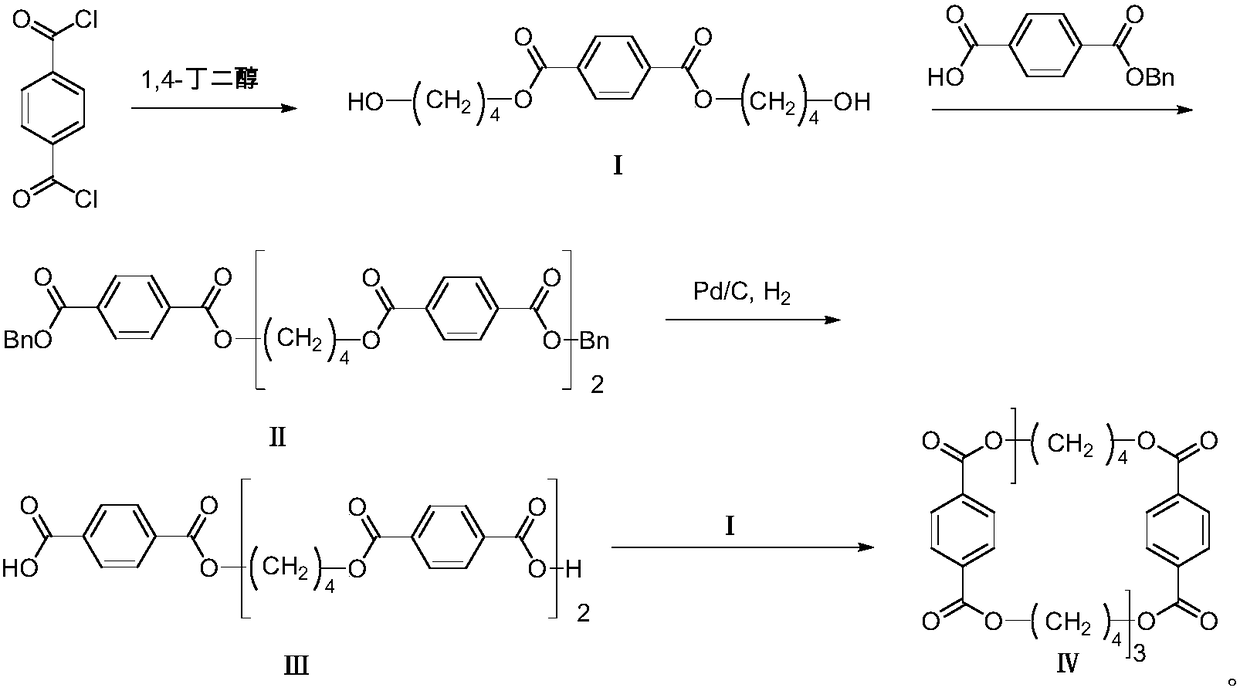
[0049] 1-1. In a single-necked bottle, add 19.7g of terephthaloyl chloride into 70mL of tetrahydrofuran, add 35g of 1,4-butanediol, drop into ice water, slowly add 23g of pyridine dropwise, and gradually rise to room temperature under nitrogen protection React for 4 hours. After the reaction is complete, add dichloromethane, wash with water, dilute hydrochloric acid and saturated sodium bicarbonate solution successively, dry over anhydrous sodium sulfate, filter, and then concentrate under reduced pressure, use (V (petroleum ether) : V (ethyl acetate) = 5 ︰1) Beating to obtain 20.3 g of bis(4-hydroxy-butyl) terephthalate, compound I, with a yield of 67%.
[0050] 1 HNMR (CDCl 3 , 400MHz), δ: 8.10(s, 4H); 4.40(t, 4H, J=6.5Hz); 3.74(t, 4H, J=6.4Hz); 1.94~1.85(m, 4H); 1.78~1.70( m, 4H).
[0051] MS (ESI), m / z: 311 [M+H] + .
[0052] 1-2. In a single-necked bottle, add 19.7g of terephthaloyl chloride into 70mL of dichloromethane, add 69.9g of 1,4-butanediol, drop into ice wa...
Embodiment 2
[0059] 2-1. In a single-necked bottle, add 6.79g of compound I and 11.2g of monobenzyl terephthalate into 100mL of dichloromethane, then add 0.67g of dimethylaminopyridine and 17.5g of EDCI, and react at room temperature under nitrogen protection 10 hours. After completion of the reaction, wash with dilute hydrochloric acid and saturated sodium carbonate solution successively, dry over anhydrous sodium sulfate, filter, then concentrate under reduced pressure, heat up and reflux with methanol for beating, and obtain 9.7g of white solid after filtration: bis(4-(4-benzyl Oxy-terephthaloyloxy) butyl) terephthalate is compound II, and the yield is 65%.
[0060] 1 HNMR (CDCl 3 , 400MHz), δ: 8.07-8.00 (m, 12H); 7.39-7.26 (m, 10H); 5.31 (s, 4H); 4.36 (s, 8H); 1.89 (s, 8H).
[0061] MS (ESI), m / z: 787 [M+H] + .
[0062] 2-2. In a single-necked bottle, add 6.79g of compound I and 14g of monobenzyl terephthalate into 100mL of chloroform, then add 0.67g of dimethylaminopyridine and 1...
Embodiment 3
[0072] 3-1. In a single-necked bottle, add 7.1g of compound II into 80mL of tetrahydrofuran, add 1.42g of 10% palladium on carbon, and 0.36g of trifluoroacetic acid, and hydrogenate at room temperature and pressure for 17 hours. After evaporating the solvent to dryness, dichloromethane was added, 2.75 g of 1,8-diazabicycloundec-7-ene (DBU) was added, and palladium carbon was removed by suction filtration. After the filtrate was concentrated under reduced pressure, 1M dilute hydrochloric acid was added to precipitate a white solid. After suction filtration, the crude product was slurried with methanol to obtain 4.18 g of white solid: 4,4'-[terephthaloylbis-(oxytetramethyleneoxycarbonyl)]dibenzoic acid, compound III, with a yield of 74% .
[0073] 1 HNMR (DMSO-d 6 , 400MHz), δ: 8.06 (d, 12H, J=4.7Hz); 4.38 (s, 8H); 1.90 (s, 8H).
[0074] 3-2. In a single-necked bottle, add 7.1 g of compound II to 80 mL of methanol, add 1.42 g of 10% palladium on carbon, and 0.71 g of trifluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com