Preparation method, product and application for TAF intermediate
An intermediate and reaction technology, applied in the field of chemical synthesis of pharmaceuticals, can solve the problems of low phenol yield, difficulty in mass production, and long reaction time, and achieve the effects of improving purity, saving time and cost, and simple and clear steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Reaction: 10.0Kg of toluene was added to the reactor, and 1.0Kg of PMPA (Tenofovir, Chinese name Tenofovir), 2.2Kg of triphenyl phosphite, 704g of triethylamine and 425g of For DMAP, heat up to 90-95°C, keep the temperature and stir for 16 hours, then cool down to 10-30°C, stop stirring and separate naturally;
[0035] 2) Post-treatment: remove the light yellow oil in the lower layer, slowly add 3Kg of 20wt% NaOH aqueous solution dropwise, add ethyl acetate for extraction (5L*3), the extraction temperature is 35-40°C, collect the lower aqueous phase, and slowly add concentrated hydrochloric acid dropwise (36-37wt%), stirred and crystallized overnight.
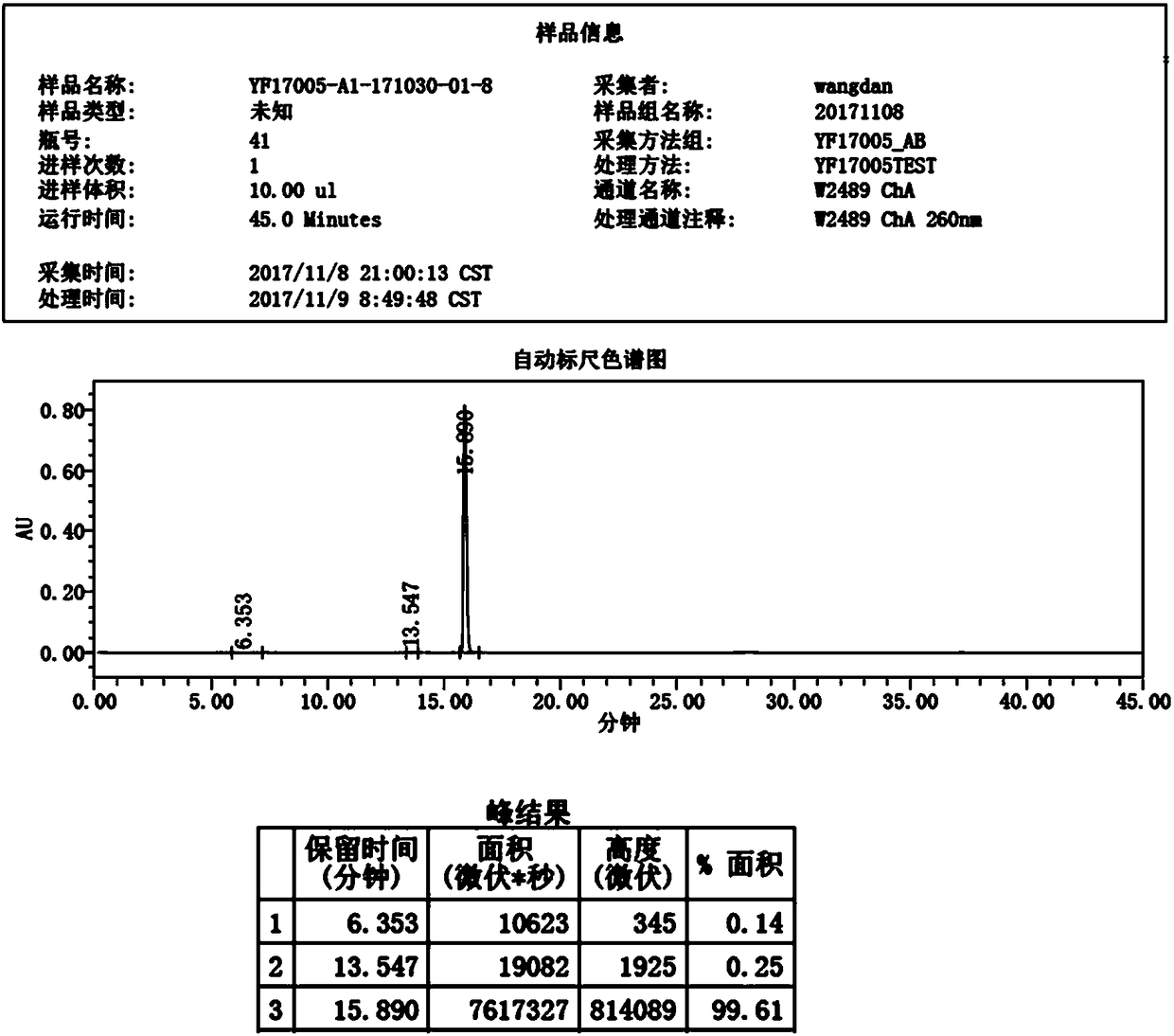
[0036] 3) After suction filtration, dry at 70-80°C for 7 hours, the moisture is less than 1.0%, the yield is 90.80%, and the purity is 99.61%. figure 1 It is the HPLC report figure of this embodiment, wherein the HPLC peak result data refer to the report figure.
Embodiment 2
[0038] 1) Reaction: The xylene of 12.0Kg is joined in the reactor, then add the triphenyl phosphite of 1.0KgPMPA, 3.0Kg, the triethylamine of 720g and the DMAP (4-dimethylamino Pyridine), heat up to 100-110°C, keep the temperature for 12 hours, then cool down to 10-15°C, stop stirring and separate naturally;
[0039] 2) Post-treatment: remove the light yellow oil in the lower layer, slowly add 3Kg of 20wt% NaOH aqueous solution dropwise, add ethyl acetate for extraction (5L*3), the extraction temperature is 40-45°C, collect the lower aqueous phase, and slowly add concentrated hydrochloric acid dropwise , stirring and crystallizing overnight.
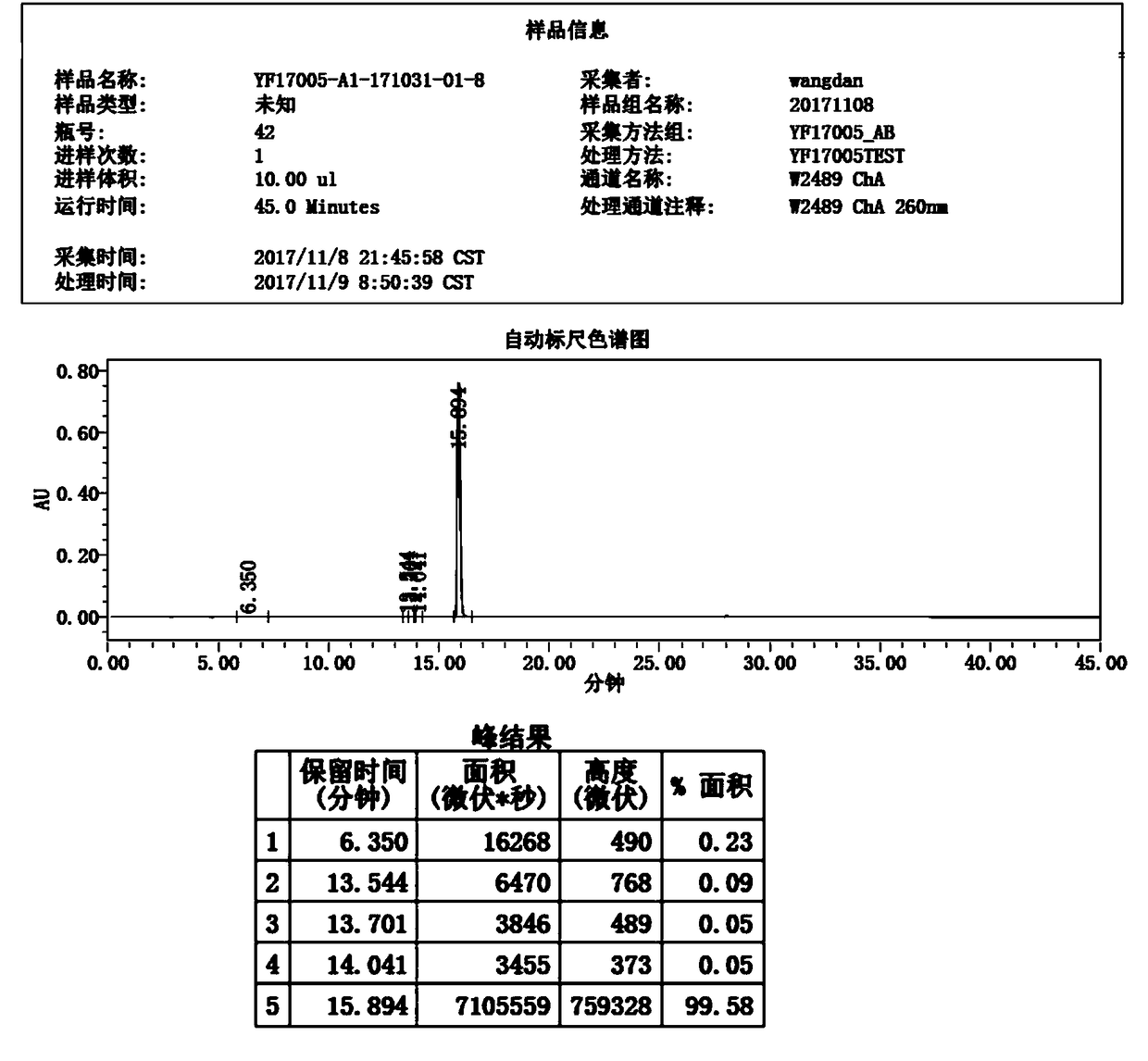
[0040] 3) After suction filtration, dry at 70-80°C for 6 hours, the moisture is less than 1.0%, the yield is 91.97%, and the purity is 99.58%. figure 2 It is the HPLC report figure of this embodiment, wherein the HPLC peak result data refer to the report figure.
Embodiment 3
[0042] 1) Reaction: Add 10Kg of toluene and 2Kg of xylene mixture into the reactor, then add 1.0KgPMPA, 3.0Kg of triphenyl phosphite, 730g of triethylamine and 450g of DMAP in a stirred state, and heat up to 100-110°C, keep the temperature for 12 hours, then lower the temperature to 10-15°C, stop stirring and separate naturally;
[0043] 2) Post-treatment: remove the light yellow oil in the lower layer, slowly add 3.2Kg of 20wt% NaOH aqueous solution dropwise, add ethyl acetate for extraction (5L*3), the extraction temperature is 40-45°C, collect the lower aqueous phase, and slowly add concentrated Hydrochloric acid, stirred and crystallized overnight.
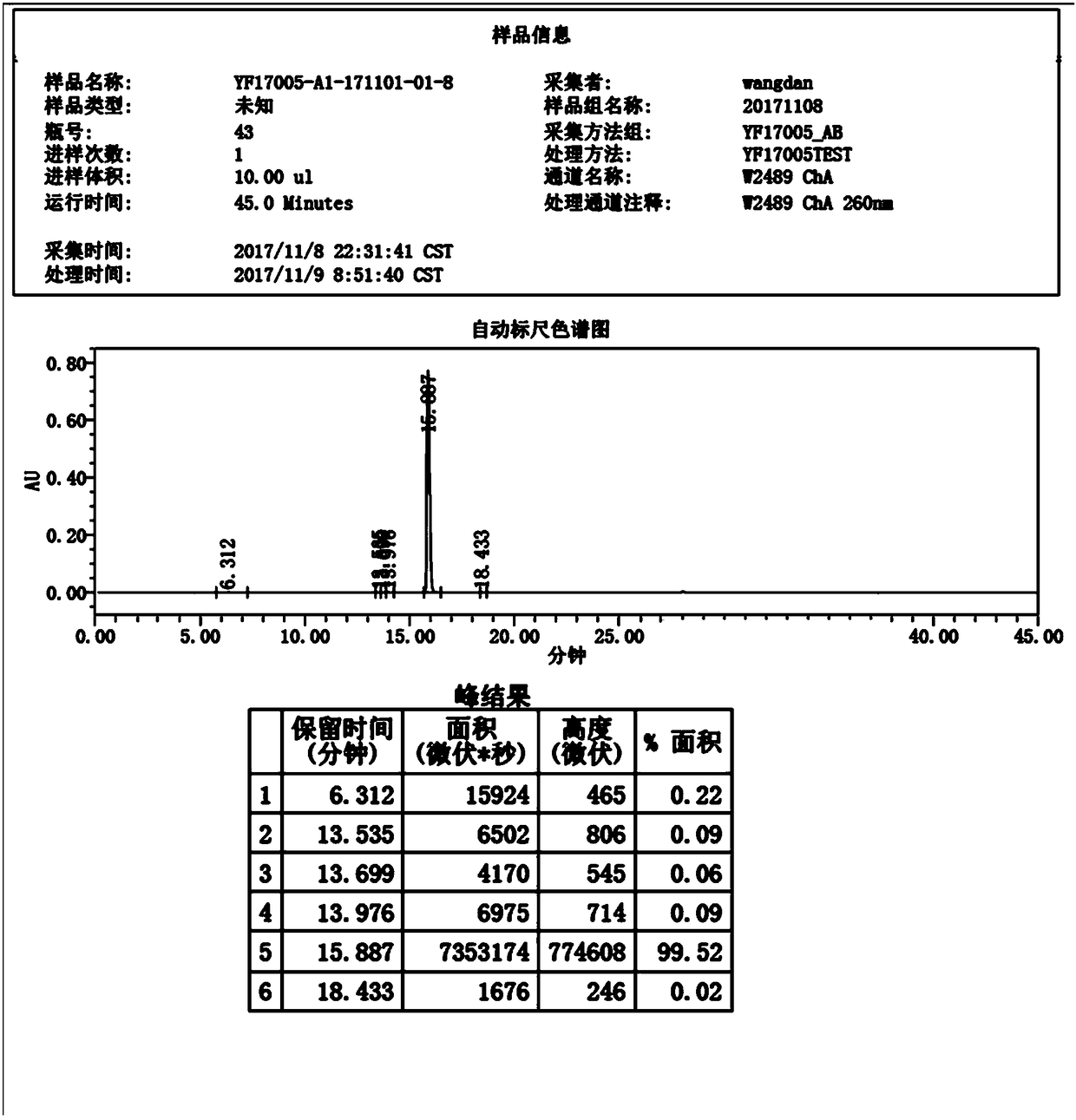
[0044] 3) Suction filtration, drying at 70-80°C for 8 hours, yield 91.54%, purity 99.52%, image 3 It is the HPLC report figure of this embodiment, wherein the HPLC peak result data refer to the report figure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com