Method for reducing self-discharge rate of high-nickel ternary lithium battery
A ternary lithium battery, self-discharge rate technology, applied in non-aqueous electrolyte battery electrodes, secondary batteries, electrode carriers/current collectors, etc. Endanger battery safety and other issues, and achieve the effect of reducing self-discharge effect and inhibiting the loss of electrode metal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
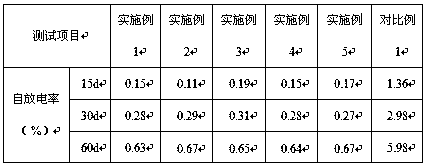
Examples
Embodiment 1
[0032] a. Add barium hydroxide and sodium hydroxide into deionized water, stir thoroughly to prepare a mixed solution;
[0033] b. Slowly drop titanium tetrachloride into the mixed solution prepared in step a, then transfer to the polytetrafluoroethylene lining, further place the lining in a hydrothermal reaction kettle, and heat for reaction;
[0034] c. After the hydrothermal reaction is completed, centrifuge, wash, dry, and heat-treat in a muffle furnace to obtain barium titanate;
[0035] d. Mix the barium titanate, lithium metal, lanthanum oxide, yttrium oxide, bismuth oxide, antimony oxide, tantalum oxide, and niobium oxide prepared in step c, and spray the mixture on the negative electrode current collector by flame spraying, then Reduce the self-discharge rate of high-nickel ternary lithium batteries.
[0036] In step d, the gas for flame spraying is acetylene.
[0037]In step a, the stirring speed is 65r / min, and the mixing time is 50min; in step b, the temperature ...
Embodiment 2
[0040] a. Add barium hydroxide and sodium hydroxide into deionized water, stir thoroughly to prepare a mixed solution;
[0041] b. Slowly drop titanium tetrachloride into the mixed solution prepared in step a, then transfer to the polytetrafluoroethylene lining, further place the lining in a hydrothermal reaction kettle, and heat for reaction;
[0042] c. After the hydrothermal reaction is completed, centrifuge, wash, dry, and heat-treat in a muffle furnace to obtain barium titanate;
[0043] d. Mix the barium titanate, lithium metal, lanthanum oxide, yttrium oxide, bismuth oxide, antimony oxide, tantalum oxide, and niobium oxide prepared in step c, and spray the mixture on the negative electrode current collector by flame spraying, then Reduce the self-discharge rate of high-nickel ternary lithium batteries.
[0044] In step d, the gas for flame spraying is propane.
[0045] In step a, the stirring speed is 50r / min, and the mixing time is 60min; in step b, the temperature o...
Embodiment 3
[0048] a. Add barium hydroxide and sodium hydroxide into deionized water, stir thoroughly to prepare a mixed solution;
[0049] b. Slowly drop titanium tetrachloride into the mixed solution prepared in step a, then transfer to the polytetrafluoroethylene lining, further place the lining in a hydrothermal reaction kettle, and heat for reaction;
[0050] c. After the hydrothermal reaction is completed, centrifuge, wash, dry, and heat-treat in a muffle furnace to obtain barium titanate;
[0051] d. Mix the barium titanate, lithium metal, lanthanum oxide, yttrium oxide, bismuth oxide, antimony oxide, tantalum oxide, and niobium oxide prepared in step c, and spray the mixture on the negative electrode current collector by flame spraying, then Reduce the self-discharge rate of high-nickel ternary lithium batteries.
[0052] In step d, the gas for flame spraying is hydrogen.
[0053] In step a, the stirring speed is 80r / min, and the mixing time is 40min; in step b, the temperature ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com