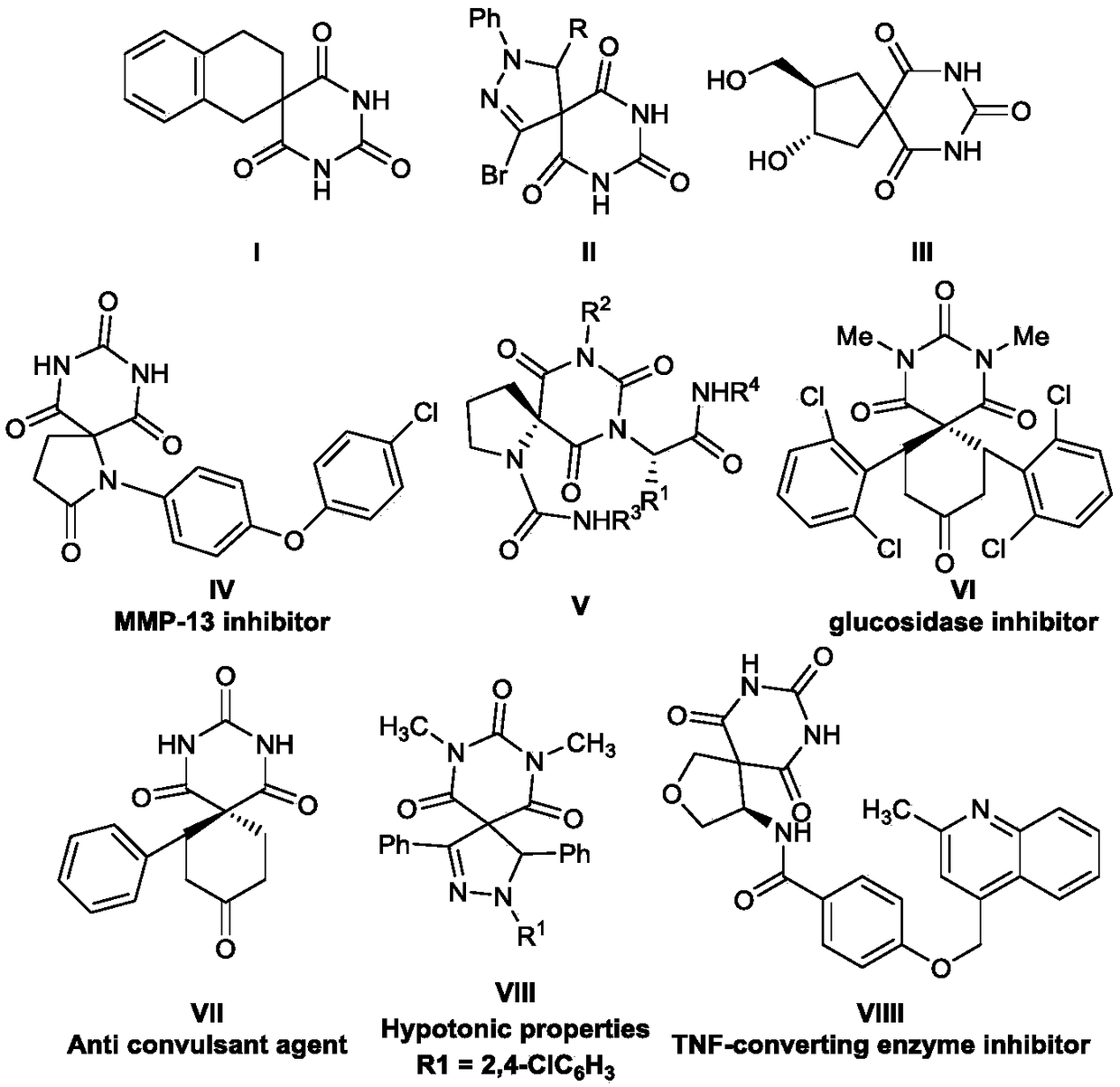
Barbituric acid-cyclohexadiene spiro compound with Boc amino and synthetic method of barbituric acid-cyclohexadiene spiro compound
A technology of aminobarbituric acid and spiro compounds, applied in the field of organic synthesis, to achieve the effects of wide application range, mild reaction conditions, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
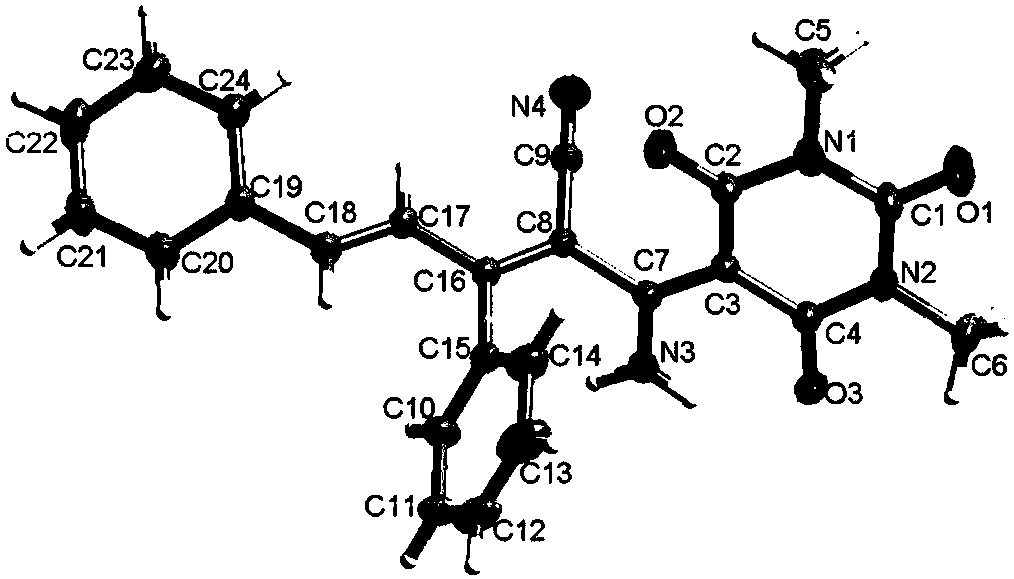
Image
Examples
Embodiment 1
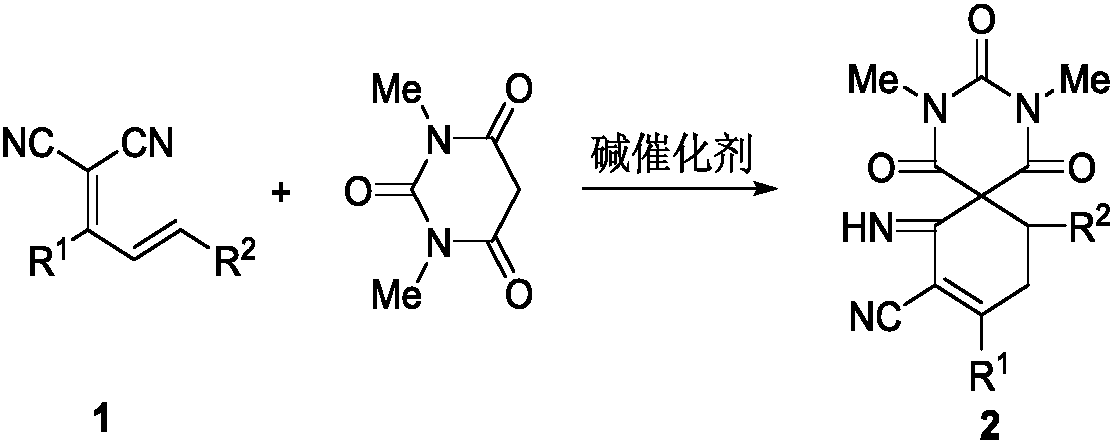
[0033] Screening of reaction conditions
[0034] Formal [5+1] cyclization of 1,1-dicyano-1,3-diene and 1,3-dimethylbarbituric acid to construct two spirobarbituric acid compounds with different structures Reaction condition screening : Take the reaction of substrate 1a and 1,3-dimethylbarbituric acid as an example:
[0035] The reaction equation is as follows:
[0036]
[0037]
[0038]
Embodiment 2
[0040] Synthesis of barbituric acid-cyclohexene spiro compound 2 with imine group
[0041]
[0042] At room temperature, 1 (0.3mmol), 1,3-dimethylbarbituric acid (0.36mmol), tetrahydrofuran (0.6mL) were successively added to the reaction flask, and then the catalyst i-Pr 2 NH(5mol%) or Et 3 N (2mol%). After the reaction was completed, the corresponding target product 2 was obtained directly through silica gel column separation (petroleum ether / ethyl acetate=10 / 1). The specific experimental data are as follows:
[0043] =12.1Hz,1H); 3.91-3.80(m,1H),3.15(s,3H),3.15(s,3H),3.01(d,J=19.1Hz,1H); 13 CNMR (75MHz, CDCl 3 ):δ=169.1, 167.3, 166.6, 165.6, 150.1, 137.4, 135.8, 131.4, 129.3, 129.2, 129.1, 127.7, 127.5, 114.3, 109.6, 62.6, 47.3, 34.7, 28.8, 28.4; , CDCl 3 ): δ=131.3, 129.2, 129.1, 129.0, 127.6, 127.5, 47.3, 34.6, 28.8, 28.4; HRMS(ESI): m / z calcd for C 24 h 21 N 4 o 3 + [M+H] + 413.1608,found: 413.1608.
[0044] 3.88-3.78(m,1H),3.18(s,3H),3.16(s,3H),2.98(d...
Embodiment 3
[0058] Synthesis of Barbituric Acid-Cyclohexadiene Spiral Compound 4 with Boc Protected Amino
[0059] Compound 3a was treated with (Boc) in dichloromethane 2 A simple treatment of O can give the barbituric acid-cyclohexadiene spiro compound 4a with a Boc-protected amino group, as shown below, with a yield of 78%. The reaction equation is as follows:
[0060]
[0061] For this reason, the conditions for the synthesis of compound 3a were firstly optimized, and the results are as follows:
[0062]
[0063]
[0064] The best condition is to use DMAP as catalyst, 95% yield to get 3a
[0065]
[0066] Characterization data of compound 3a:
[0067] (2E,4E)-2-(amino(1,3-dimethyl-2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)methyl)-3,5-diphenylpenta-2,4-dienenitrile(3a) Synthesis: Experimental steps: Add 1,1-dicyano-1,3-diene 1a and 1,3-dimethylbarbituric acid, catalyst, and solvent to the reaction flask in sequence, stir at room temperature, and monitor by TLC , 1a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com