Application of Protegrin-1 antibacterial peptide derivative in preparation of drugs resisting oral pathogens
A technology of pathogenic bacteria and drugs, which is applied in the field of preparing anti-oral pathogenic bacteria drugs, can solve the problems of oral mucosa irritation, heavy bitter taste, and taste changes, and achieve better therapeutic effects, good application prospects, and clear drug effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The preparation of embodiment 1 anti-oral oral pathogenic bacteria medicine of the present invention
[0067] Weigh 60-500mg of the Protegrin-1 antimicrobial peptide derivative shown in any one of SEQ ID NO.1~SEQ ID NO.8, 600-5000mg of polylysine, and then weigh one or more stabilizers (according to the types and proportions in Table 4), the components are dissolved in distilled water, pre-frozen at -20°C, frozen at -80°C, and freeze-dried to obtain a condensed mixture of functional ingredients and stabilizers. Then this mixture is dissolved with distilled water, and the volume is adjusted to 1 L, and the spray for treating oral diseases is obtained.
Embodiment 2
[0068] Embodiment 2 Preparation of anti-oral pathogenic bacteria medicine of the present invention
[0069] Weigh 40 mg of the Protegrin-1 antimicrobial peptide derivative shown in SEQ ID NO.1 to SEQ ID NO.8, 440 mg of polylysine, then weigh 10 mg of cysteine and 80 mg of valine, and mix the components After dissolving in distilled water, it was pre-frozen at -20°C, frozen at -80°C, and freeze-dried to obtain a mixture. Then this mixture is dissolved with distilled water, and the volume is adjusted to 1 L, and the spray for treating oral diseases is obtained.
Embodiment 3
[0070] Example 3 Investigation of the killing effect of the polypeptide of the present invention on oral pathogenic bacteria
[0071] 1. Preparation of different peptide samples
[0072] Polylysine, Protegrin-1, polypeptides shown in SEQ ID NO.1-SEQ ID NO.10 and their complexes were used as samples respectively to study the effect of different polypeptides on oral pathogenic bacteria.
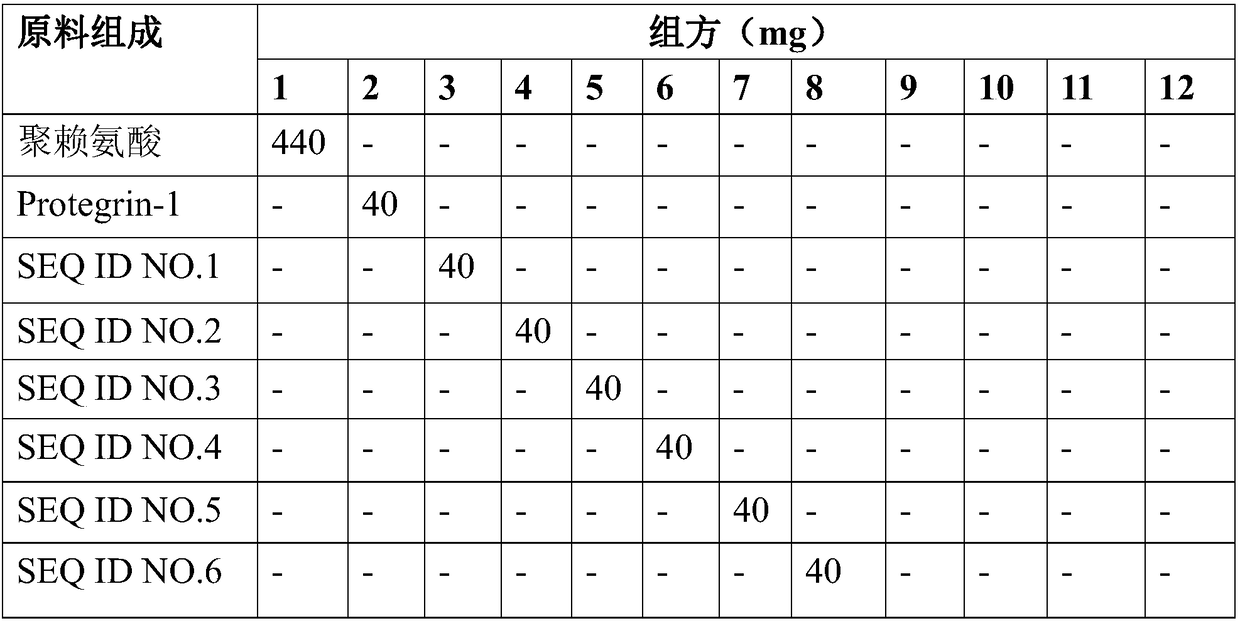
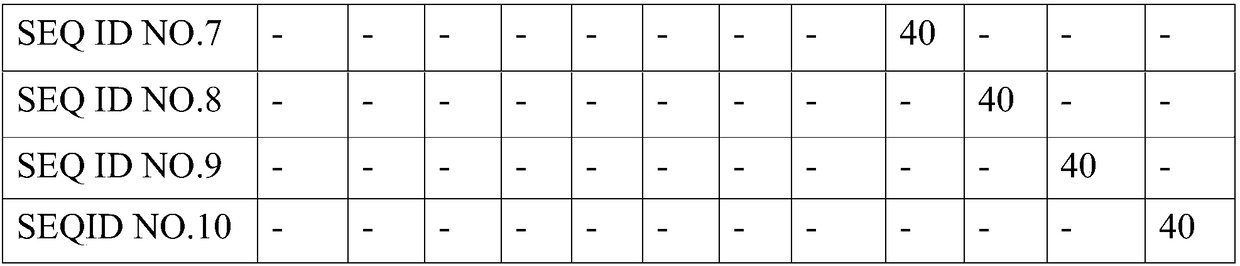
[0073] The drug formulation is shown in Table 1.
[0074] Table 1 Recipe of different polypeptide samples
[0075]
[0076]
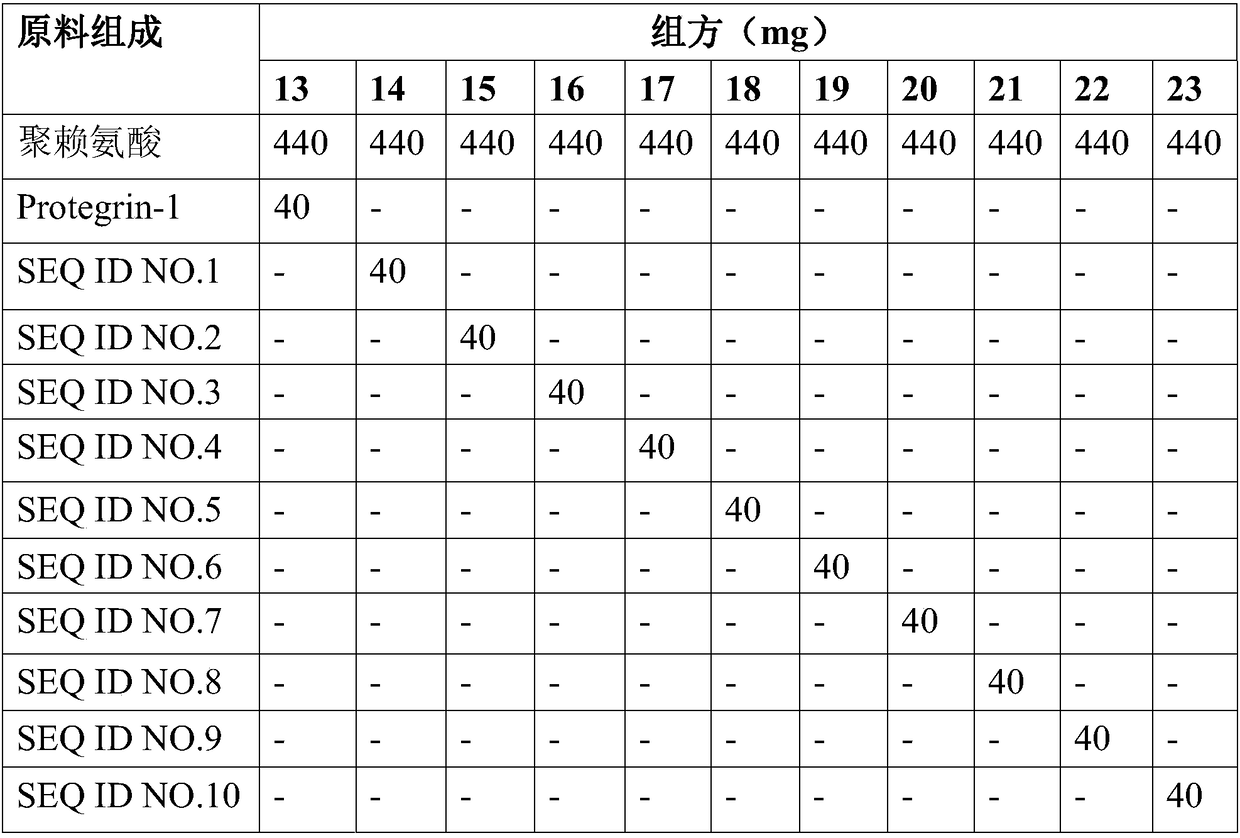
[0077] Table 1 (continued) Recipe of different polypeptide samples
[0078]
[0079] Remark:
[0080] The sequence of Protegrin-1 is: ArgGlyGlyArgLeuCys Tyr CysArgArgArgPheCys Val CysVal GlyArg;
[0081] h 2 N-RGGRLCYCRRRFCVCVGR-CONH 2
[0082] The sequence of SEQ No.9 is:
[0083] h 2 N-Arg-Gly-Gly-Leu-{D-Cys}-Tyr-Cys-Arg-Gly-Arg-Phe-Cys-Val-Cys-Val-Gly-Arg-CONH 2 ;
[0084] h 2 N-RGGLCYCRGRFCVCVGR-CONH 2
[0085] The sequence of SEQ No.10 is:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com