Tetraphenylethylene blue-fluorescence organic compound, preparation method thereof and blue organic electroluminescence device
An organic compound, blue fluorescence technology, applied in the field of materials, can solve the problems of unfavorable carrier injection and transmission, low device efficiency, etc., and achieve the effect of inhibiting exciton annihilation, good thermal stability, and strong fluorescence emission.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The invention provides a preparation method of a tetraphenylethylene blue fluorescent organic compound, comprising:
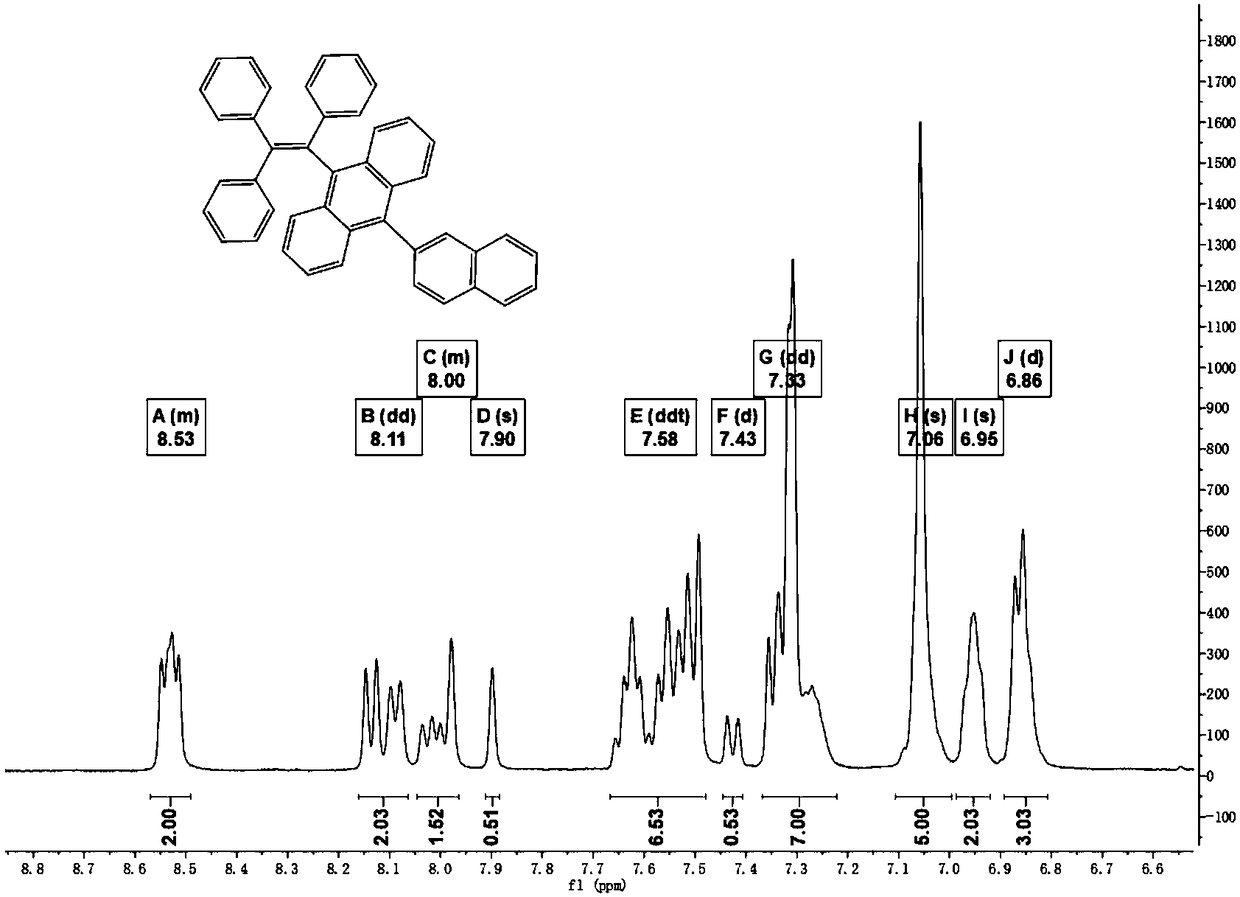
[0041] One of 9-(1-naphthyl)-10-anthracene boronic acid and 9-(2-naphthyl)-10-anthracene boronic acid is reacted with triphenylbromoethylene to obtain structure shown in formula (I) compound;
[0042]
[0043] Wherein, Ar is naphthyl.
[0044] In the present invention, one of 9-(1-naphthyl)-10-anthraceneboronic acid and 9-(2-naphthyl)-10-anthraceneboronic acid is reacted with triphenylbromoethylene specifically as follows: 9 One of -(1-naphthyl)-10-anthraceneboronic acid and 9-(2-naphthyl)-10-anthraceneboronic acid is mixed with triphenylbromoethylene, catalyst and base, and heated under inert gas conditions The reaction was refluxed to obtain a solid product.
[0045] Wherein, the catalyst of the reaction is preferably tetrakis(triphenylphosphine) palladium; the base of the reaction is preferably potassium carbonate; the inert gas includes but not...
Embodiment 1
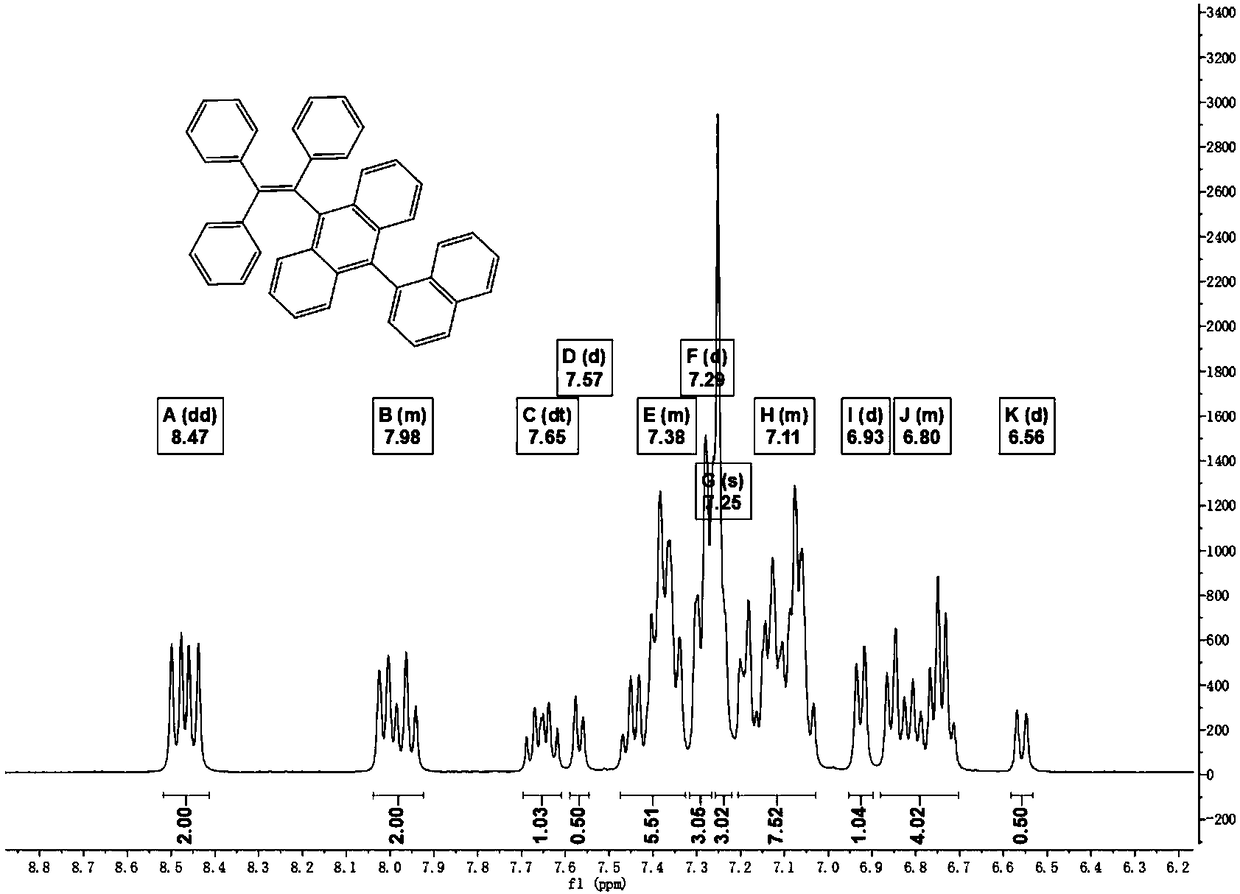
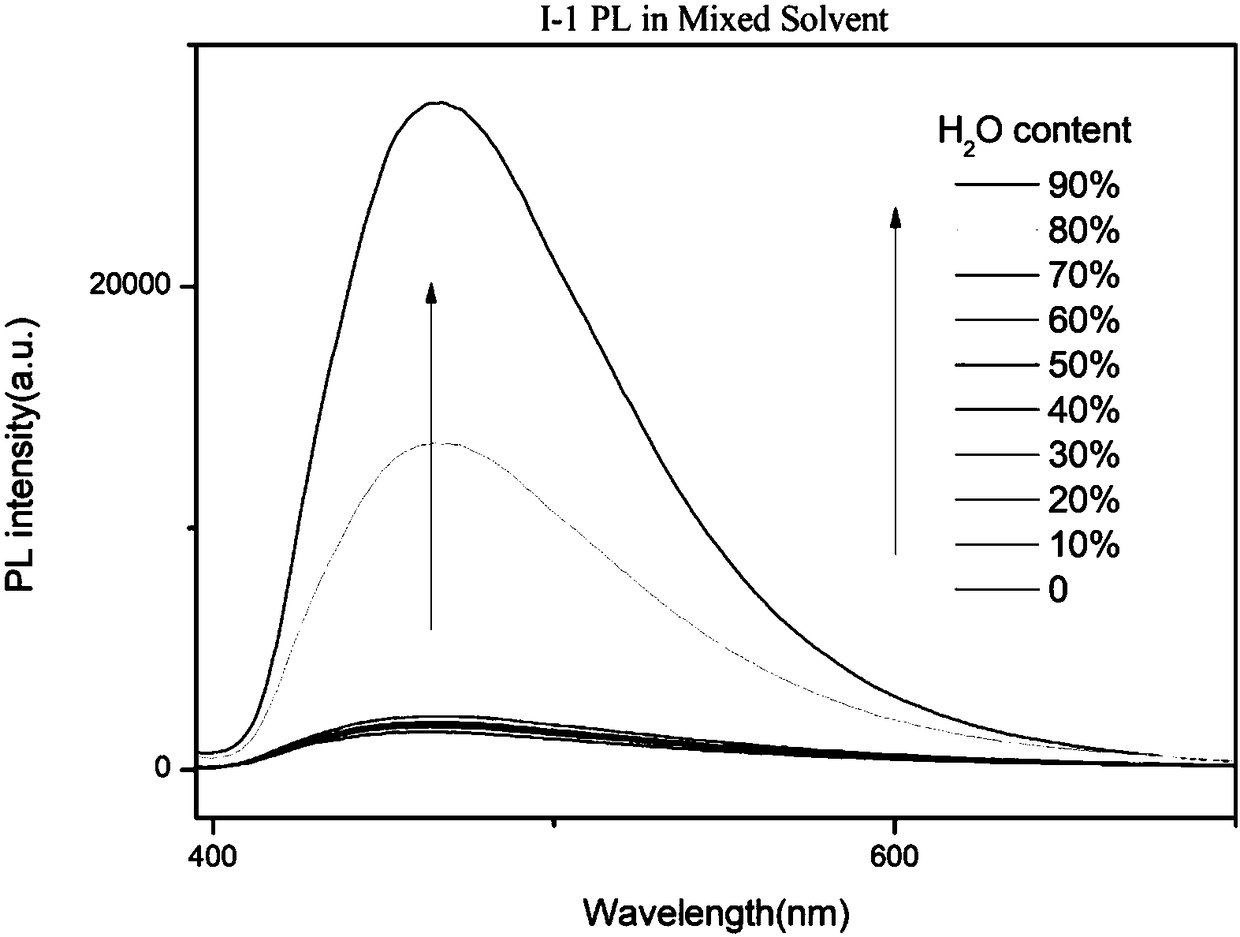
[0072] A: Preparation of compound (I-1)
[0073] Weigh 1.0g of 9-(1-naphthyl)-10-anthracenboronic acid, 1.25g of triphenylbromoethylene, 1.59g of potassium carbonate, 50mL of ethylene glycol dimethyl ether and 5mL of water into a 100mL two-necked flask. Stir to remove the air in the device and protect it with nitrogen, then add 99.6 mg of tetrakis(triphenylphosphine)palladium under nitrogen flow. Heated to 80°C, condensed and refluxed for 30 hours. As the reaction progressed, the solution turned from light yellow to orange-brown. The crude product solution was extracted with dichloromethane and water, and the organic layer was dried over anhydrous magnesium sulfate and filtered. The crude product obtained after the filtrate was distilled under reduced pressure was separated with a silica gel column, and the eluent was a mixed solvent composed of dichloromethane and petroleum ether with a volume ratio of 1:8. The resulting pure product solution was distilled under reduced pr...
Embodiment 2
[0077] OLED device fabrication
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com