Imidazole-2-thioketone compound preparation method
An ester compound and compound technology are applied in the field of preparation of imidazole-2-thione compounds and can solve problems such as complicated operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The invention provides a kind of preparation method of imidazole-2-thiones compound, comprises the following steps:
[0044] mixing amidine compounds, isothiocyanate compounds, α-halogenated ketone compounds and an organic solvent to obtain a raw material mixture;
[0045] adjusting the pH value of the raw material mixture to alkaline to obtain an alkaline reaction solution;
[0046] The alkaline reaction solution undergoes a series cyclization reaction under heating conditions to obtain imidazole-2-thione compounds.
[0047] The invention mixes amidine compounds, isothiocyanate compounds, alpha-halogenated ketone compounds and organic solvents to obtain a raw material mixture. In the present invention, the structural formula of the amidine compound is preferably R 2 NH=CNH 2 , the R 2 Preferred are phenyl, alkyl-substituted phenyl, alkoxy-substituted phenyl, trifluoromethyl-substituted phenyl, halogen-substituted phenyl, cycloalkyl, alkyl or amine groups. In the p...
Embodiment 1
[0068] Mix 0.1mmol benzamidine, 0.1mmol phenyl isothiocyanate, 0.3mmol ethyl 2-bromoacetate, 0.3mmol sodium hydroxide and 1mL acetonitrile, heat at 70°C, stir for 12h to carry out a series cyclization reaction; After the series cyclization reaction is finished, the resulting material is subjected to column chromatography purification treatment (the eluent used is a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 5:1), and the gained eluent is spin-dried to obtain the product, The yield was 52% and the purity was 99.9%.
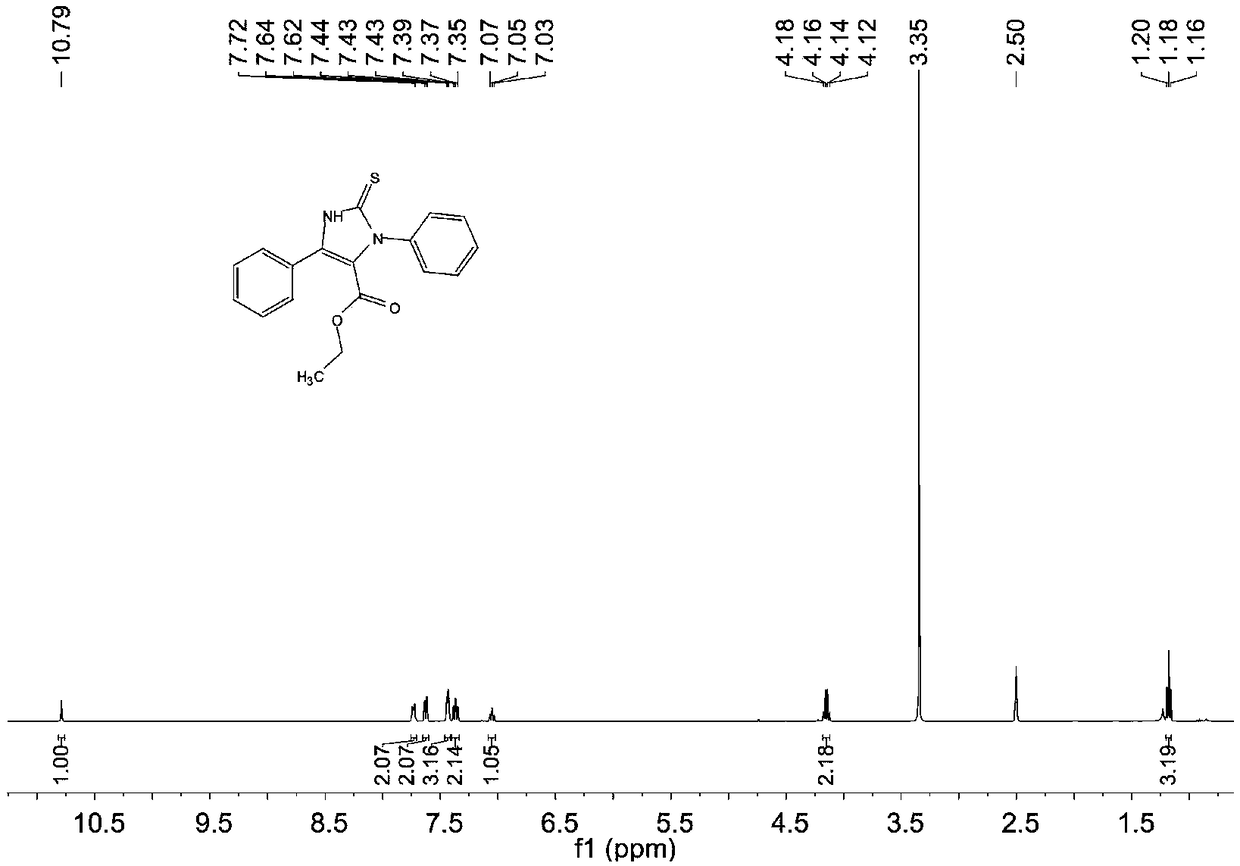
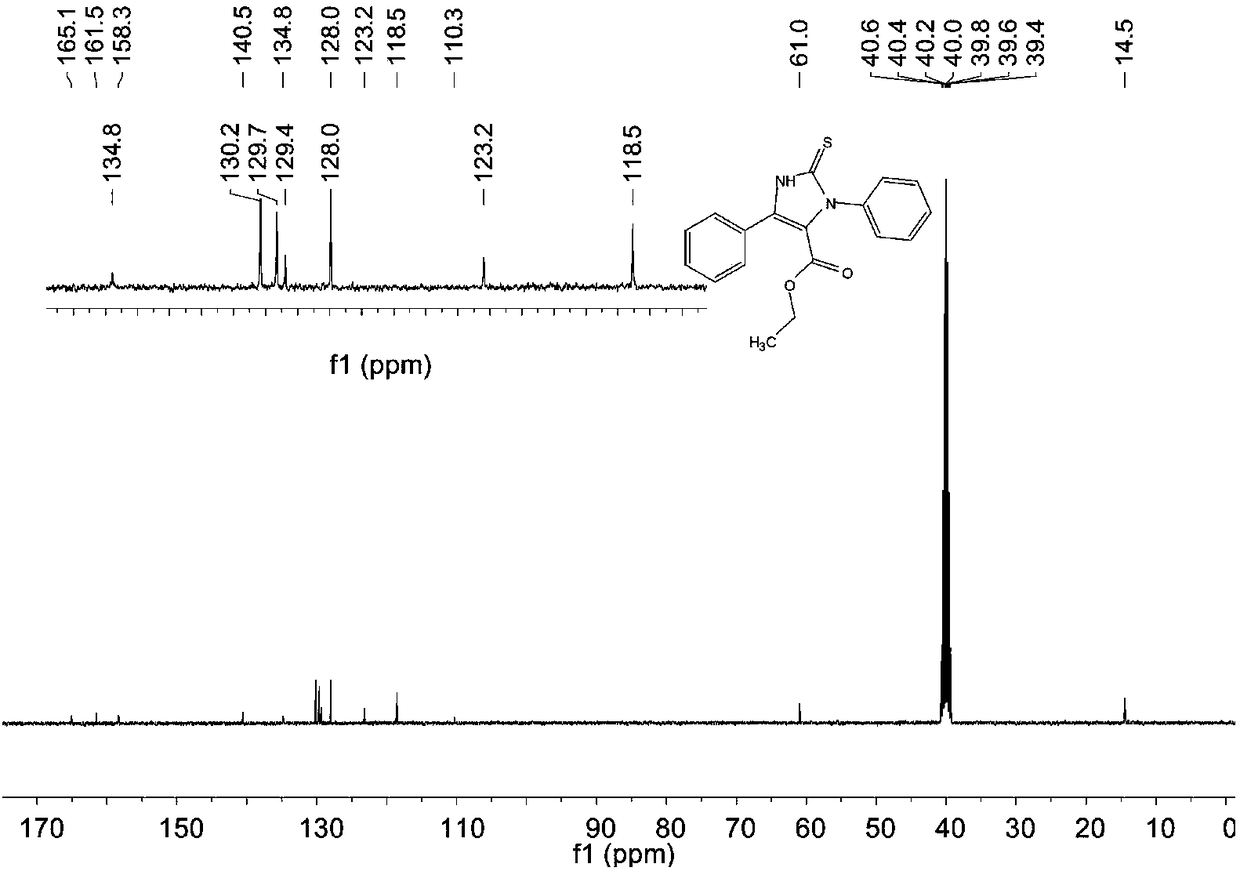
[0069] The structure of the obtained product is characterized, and the proton nuclear magnetic resonance spectrum and the carbon nuclear magnetic resonance spectrum are respectively as follows figure 1 and figure 2 As shown, the structural characterization data are as follows:
[0070] 1 H NMR (400MHz, DMSO): δ=10.79(s, 1H), δ=7.74-7.72(m, 2H), δ=7.64-7.62(d, J=8Hz, 2H), δ=7.44-7.43(t ,J=4Hz,3H),δ=7.39-7.35(t,J=16Hz,2H),δ=7.07-7....
Embodiment 2
[0076] Mix 0.1mmol p-trifluoromethyl benzamidine, 0.1mmol phenyl isothiocyanate, 0.3mmol ethyl 2-bromoacetate, 0.3mmol sodium hydroxide and 1mL acetonitrile, heat at 70°C, stir for 12h to carry out series ring reaction; after the completion of the series cyclization reaction, the gained material is subjected to column chromatography purification treatment (the eluent used is a mixed solvent of sherwood oil and ethyl acetate with a volume ratio of 5:1), and the gained eluent is vortexed Drying yielded the product in 59% yield and 99.8% purity.
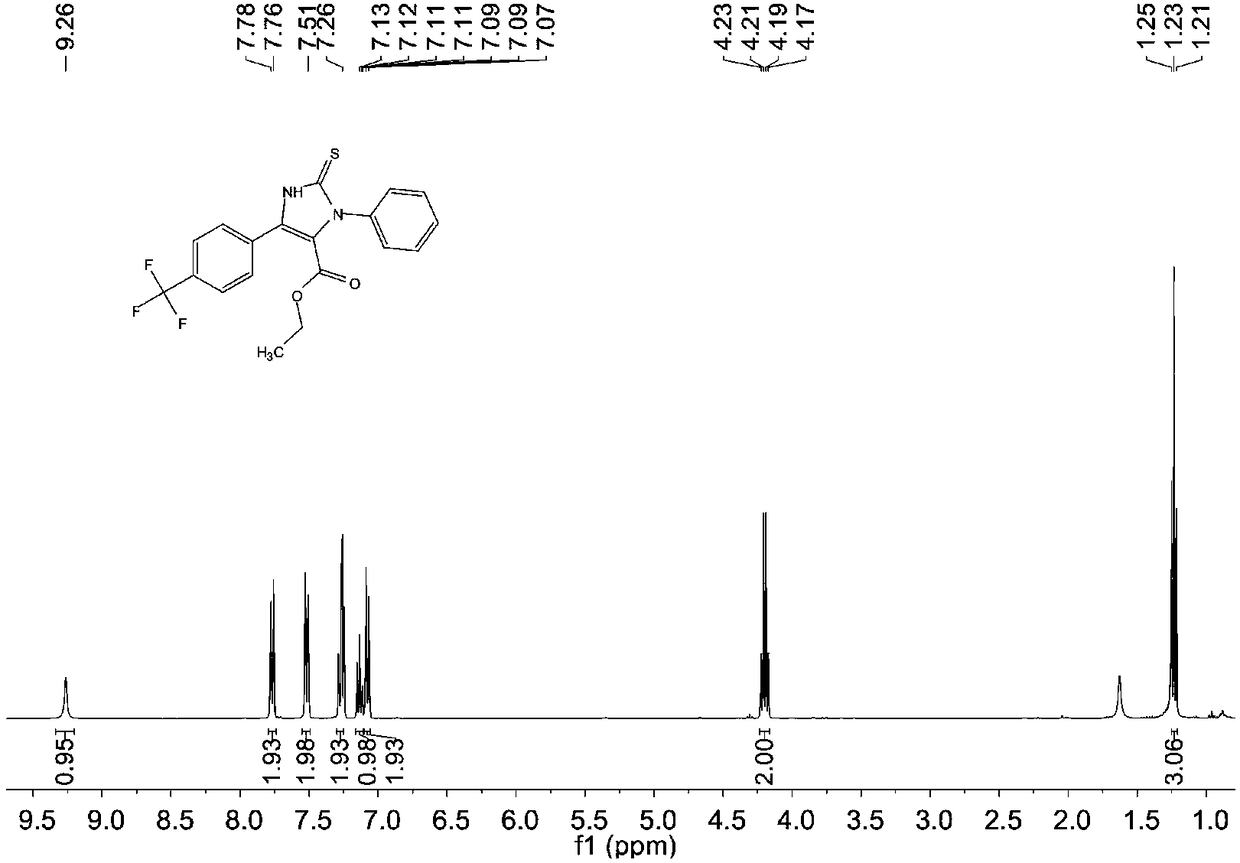
[0077] The structure of the obtained product is characterized, and the proton nuclear magnetic resonance spectrum and the carbon nuclear magnetic resonance spectrum are respectively as follows image 3 and Figure 4 As shown, the structural characterization data are as follows:
[0078] 1 H NMR (400MHz, CDCl 3 ): δ=9.26(s, 1H), δ=7.78-7.76(d, J=8Hz, 2H), δ=7.53-7.51(d, J=8Hz, 2H), δ=7.269-7.25(m, 2H ),δ=7.15-7.11(m,1H),δ=7.09-7.07(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com