a releasable so 2 Polymers, their preparation methods and applications, and nanomicelles
A polymer, SO2 technology, applied in the direction of drug combination, sulfur/selenium/tellurium active ingredients, emulsion delivery, etc., can solve the problem that the effect of cancer treatment has not been studied, and achieve the improvement of active oxygen level and obvious killing effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The present invention provides a SO described in the technical solution 2 The preparation method of polymer, comprises the following steps:
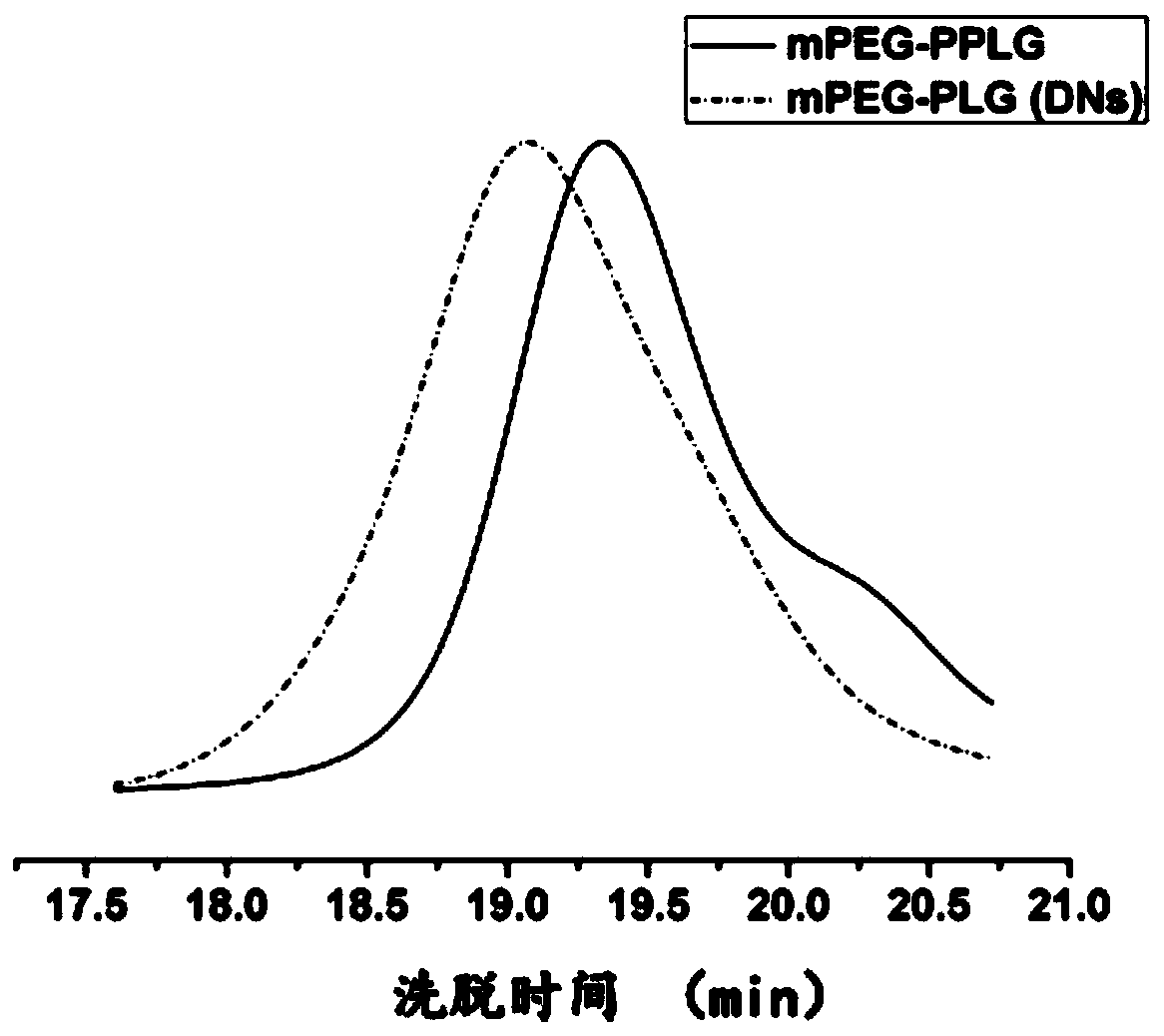
[0044]In the presence of a catalyst and a solvent, the polyethylene glycol monomethyl ether-poly(γ-propargyl-glutamic acid ester) block copolymer having the structure of formula II and the 3-azidopropene having the structure of formula III The base-2,4-dinitrobenzenesulfonamide is reacted by click chemistry to obtain SO with the structure of formula I 2 polymer;
[0045]
[0046] In formula II, 10≤m≤450; 5≤n≤100;
[0047]
[0048] In the present invention, in order to distinguish it from the catalyst in the following technical scheme, the polyethylene glycol monomethyl ether-poly(γ-propargyl-glutamic acid ester) block copolymer with formula II structure and the The catalyst adopted by the 3-azidopropyl-2,4-dinitrobenzenesulfonamide with the structure of formula III through the click chemical reaction is named as the first...
Embodiment 1
[0073] Embodiment 1: the synthesis of 3-azidopropylamine
[0074] Weigh 8.8 g of 3-chloropropylamine hydrochloride and 13.3 g of sodium azide, and add 200 mL of water into the reaction flask. The reaction was stirred at 80°C overnight, and the resulting mixture was adjusted to pH 11 with potassium hydroxide, and then extracted three times with 100 ml of ether. The organic phase was collected and dried over anhydrous magnesium sulfate, and the diethyl ether was removed by rotary evaporation to obtain 3-azidopropylamine as a light yellow oily product.
Embodiment 2
[0075] Embodiment 2: the synthesis of 3-azidopropyl group-2,4-dinitrobenzenesulfonamide with formula III structure
[0076] Weigh 2.0 g of 3-azidopropylamine prepared in Example 1, and dissolve 3.3 g of sodium bicarbonate in 20 ml of anhydrous THF. Weigh 5.5 g of dinitrobenzenesulfonyl chloride, dissolve it in 20 mL of anhydrous THF, slowly add it dropwise and stir in an ice bath, react for 24 hours, acidify the pH value to 1 with hydrochloric acid, and then extract three times with 50 mL of dichloromethane , the organic phase was collected and dried with anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation to obtain the yellow-brown product 3-azidopropyl-2,4-dinitrobenzenesulfonamide.
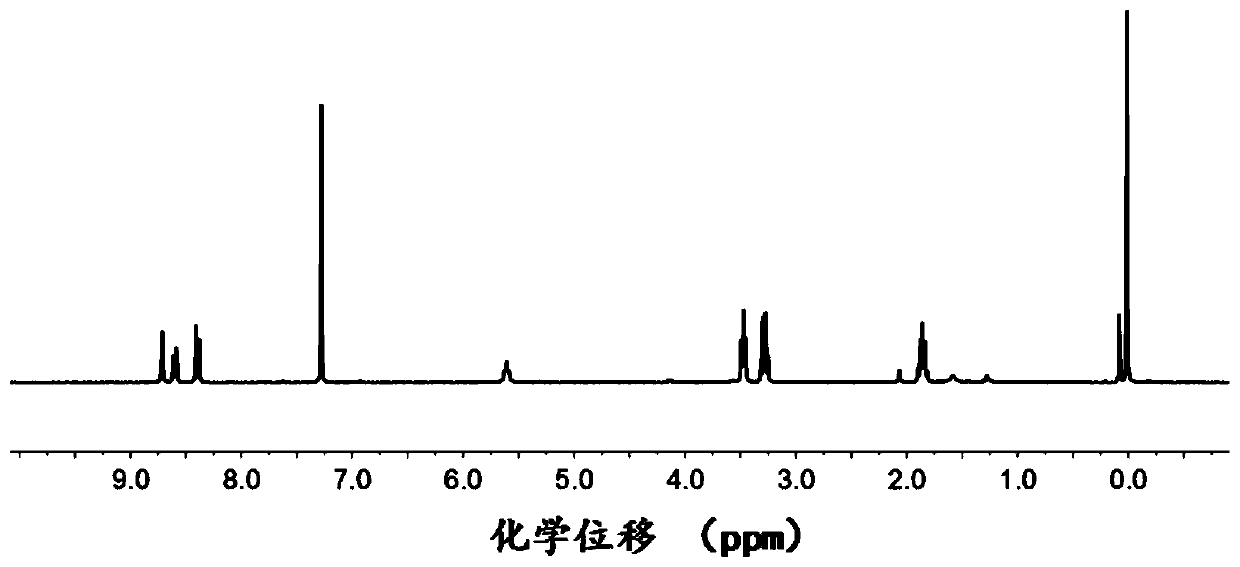
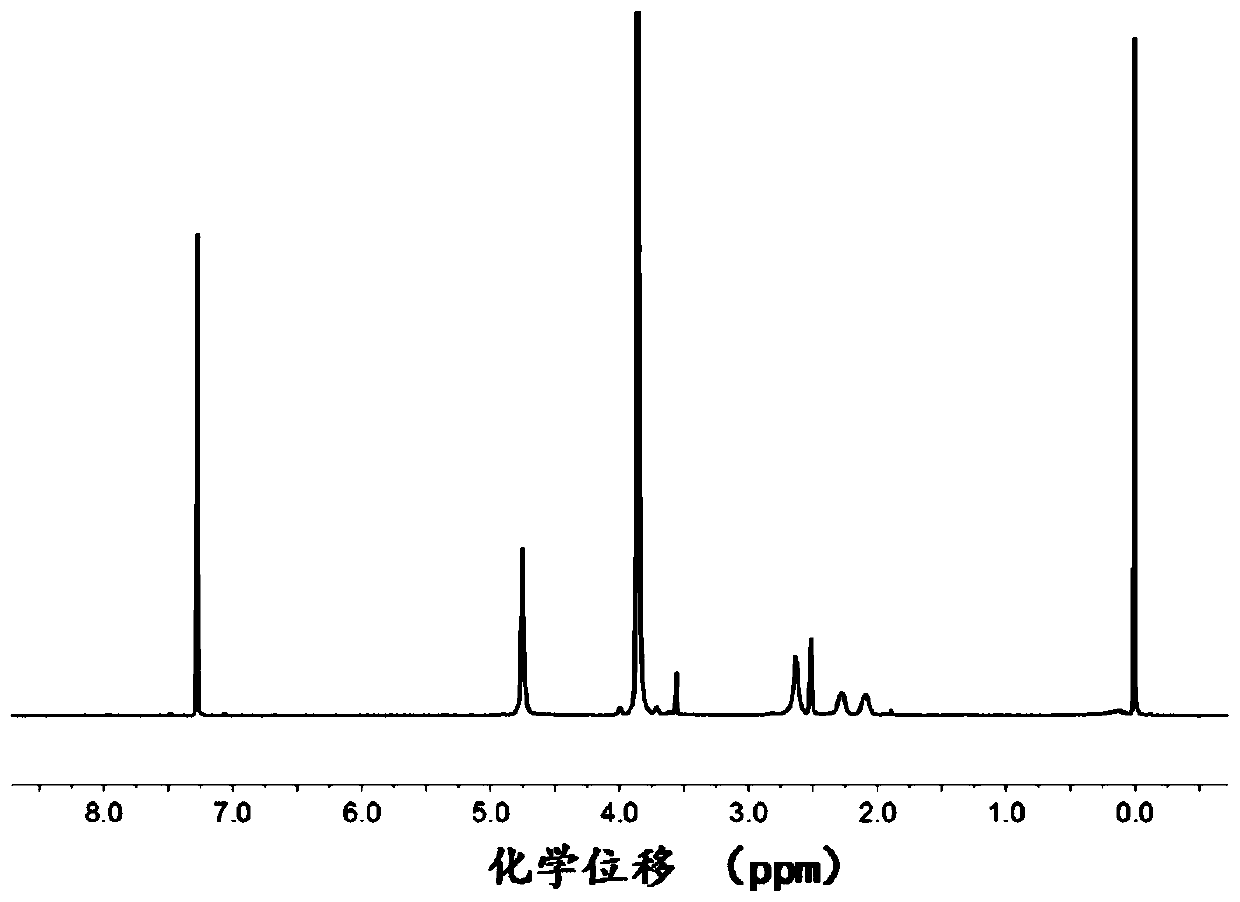
[0077] The 3-azidopropyl-2,4-dinitrobenzenesulfonamide obtained above is detected, figure 2 The proton nuclear magnetic resonance spectrum of the 3-azidopropyl-2,4-dinitrobenzenesulfonamide prepared in Example 2 of the present invention; the results show that the 3-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com