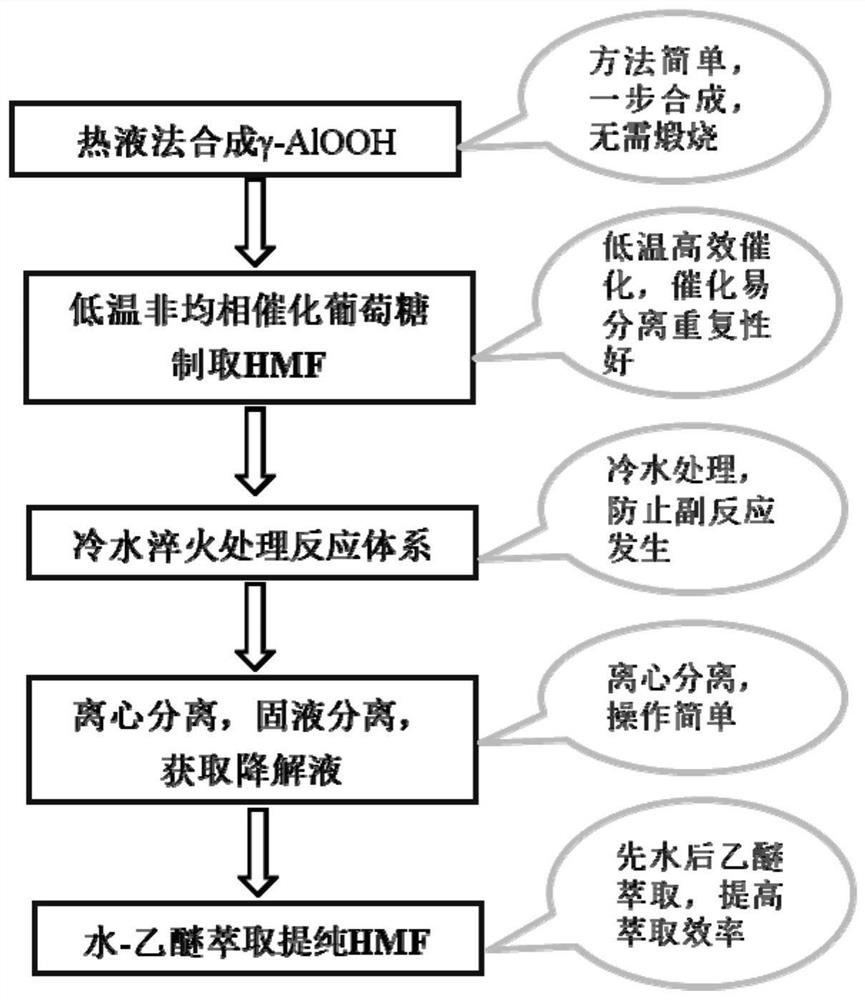
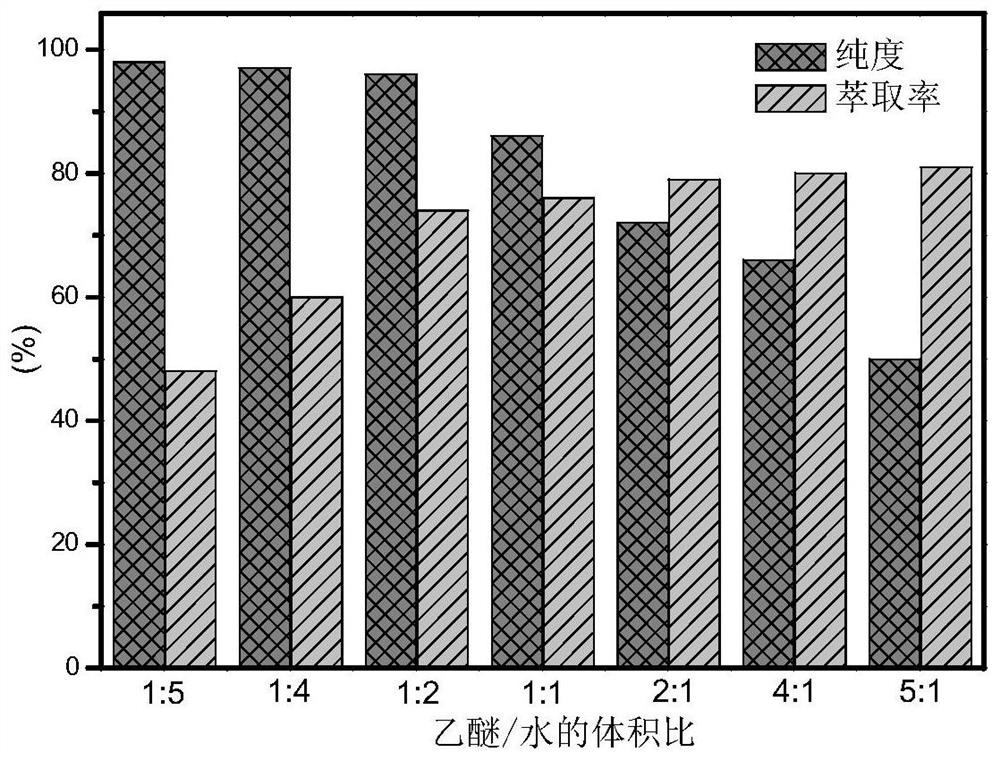
A method for preparing 5-hydroxymethylfurfural from glucose catalyzed by boehmite at low temperature
A technology of hydroxymethyl furfural and boehmite, applied in the directions of organic chemistry, chemical recovery, etc., can solve the problems of high reaction temperature, complicated catalyst preparation, etc., achieves simple preparation method, good industrial application prospect, and avoids a large number of side reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
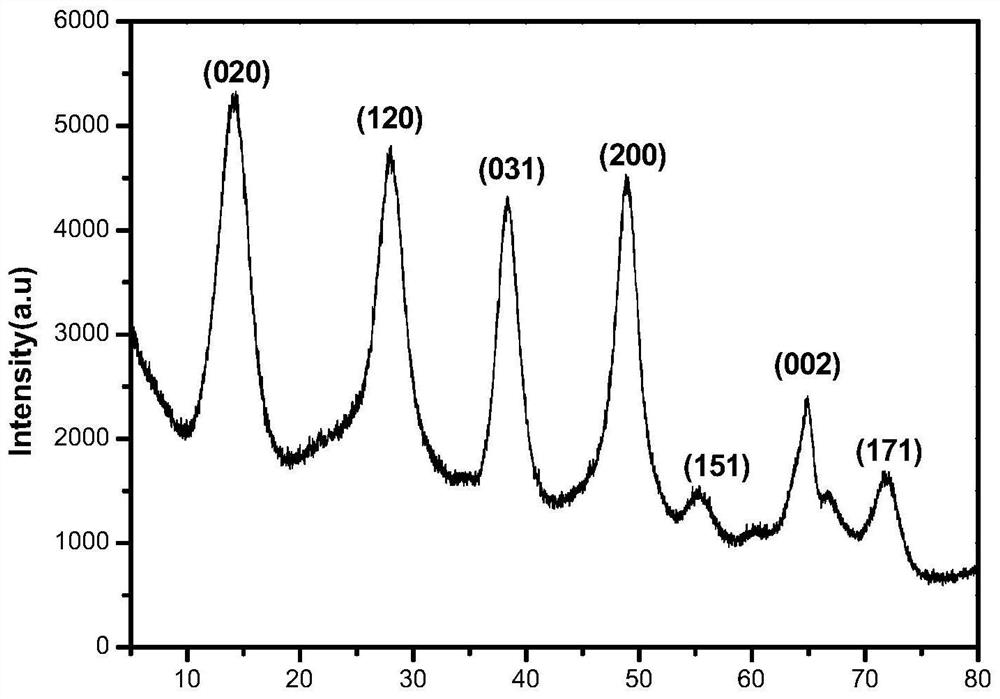
[0032] The 30mmol NH 4 HCO 3 And 15mmol Al (NO 3 ) 3The addition of 50 ml of deionized water in strong agitation. After it turns into a clear transparent homogeneous solution, 25% of the concentrated aqueous solution is slowly added to adjust the pH to 9. After it turned into a uniform mixture, it was transferred to 100 mL of the reactor of the inner lining polytetrafluoroethylene, and at 150 ° C for 12 h. When the reaction time reaches the set time, the reactor is removed. Naturally, after cooling to room temperature, the reactor is opened, and the separation of the catalyst and washing work is performed, and the solid product is obtained. Finally, the solid product was dried at 150 ° C for 12 h, i.e., obtained solid catalyst γ-Alooh. This solid catalyst was analyzed by XRD. like figure 2 As shown, from figure 2 It can be seen that all diffraction peaks of the sample are consistent with the orthogonal γ-AlOOH (JCPDS 021-1307) marker diffraction peak, and no other substance diffra...
Embodiment 2
[0036] The γ-AlOOH preparation method and Example 1 were identical. Glucose conversion is also the same as in Example 1.
[0037] Dimethyl sulfoxide (DMSO) was increased to 2.0 g during the preparation of 5-hydroxymethylfurifer. The specific process is as follows: 0.1 g of glucose and 0.1 g of solid catalyst γ-AlOOH were added to 2.0 g of dimethyl sulfoxide, and the above solution was transferred to a 130 ° C oil bath, and the magnetic force was stirred. After the reaction was completed, 20 ml of cold deionized water was added to the reaction mixture for quenching treatment. The re-use centrifuge was separated at a speed of 10000 rpm, and the upper liquid was collected, and the degradation of HMF was obtained. The little degradation solution was taken, 300 times diluted with deionized water, and then analyzed using the product in the degradation liquid using high performance liquid chromatography, and the HMF yield of the reaction was 60% and HMF selectivity of 63%, glucose conver...
Embodiment 3
[0039] The γ-AlOOH preparation method and Example 1 were identical. Glucose conversion is also the same as in Example 1.
[0040] The amount of dimethyl sulfoxide (DMSO) was reduced to 0.5 g during the preparation of 5-hydroxymethyl furfural. The specific process is as follows: 0.1 g of glucose and 0.1 g of solid catalyst γ-AlOOH were added to 0.5 g of dimethyl sulfoxide, and the above solution was transferred to a 130 ° C oil bath, and the magnetic force was stirred. 3 h. After the reaction was completed, 20 ml of cold deionized water was added to the reaction mixture for quenching treatment. After the centrifuge was separated at a speed of 10000 rpm, the upper liquid was collected, and the degradation of 5-hydroxyhydehydehyde was obtained. The little degradation solution was taken out, diluted with deionized water, and then analyzed the product in the degradation liquid using high performance liquid chromatography, calculated that the HMF yield of the reaction was 45% and HMF se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com